Physics Heat Transfer and Thermodynamics 1 Heat and


- Slides: 14

Physics Heat Transfer and Thermodynamics 1

Heat and Thermodynamics • • • Conservation of Energy Heat Flow in a System Heat Transfer Conduction, Convection, and Radiation Chemical Energy Physical and Chemical Changes 2

The Conservation Of Energy • The amount of energy in a closed system remains constant and energy is neither created nor destroyed. Energy can be converted from one form to another (potential energy can be converted to kinetic energy) but the total energy within the system remains the same. • Δ in form but no Δ in the amount of energy It’s the first Law of Thermodynamics! 3 Δ= change

Types of Energies 1. Kinetic-energy of motion 2. Thermal-energy of moving particles, measured as temperature of a system 3. Sound-energy of a mechanical wave moving through a medium 4. Electromagnetic (light)-electric and magnetic energy moving together through space (vacuum or low density medium) 4

Types of Energies 5. Potential-stored energy, or energy of position 6. Gravitational-type of potential energy due to position of an object with respect to another object. 7. Elastic-type of potential energy due to the change in form (bending or stretching) of an elastic object like a spring. 8. Chemical-energy stored in the bonds between atoms or molecules. 5

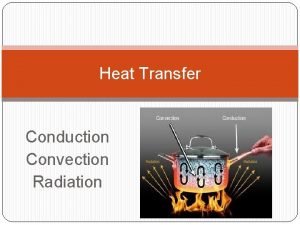
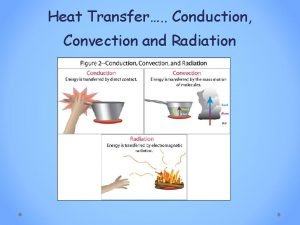
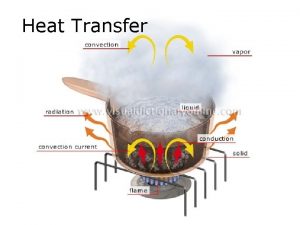
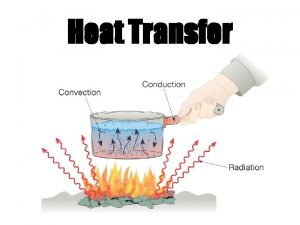
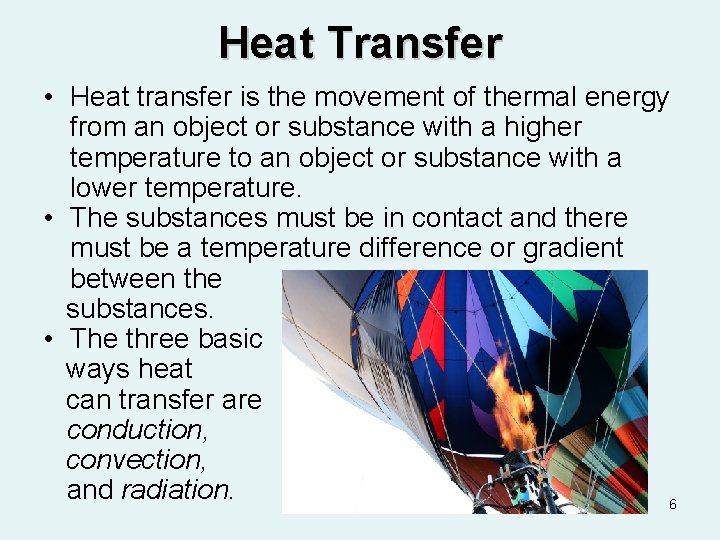
Heat Transfer • Heat transfer is the movement of thermal energy from an object or substance with a higher temperature to an object or substance with a lower temperature. • The substances must be in contact and there must be a temperature difference or gradient between the substances. • The three basic ways heat can transfer are conduction, convection, and radiation. 6

Heat Transfer and Temperature • Temperature is the measure of the average energy of motion of the particles in a substance. (kinetic energy, thermal energy) • When the particles in a substance increase their movement (kinetic energy) the temperature of that substance has increased. When the particles move slower, the temperature of the substance has decreased. 7

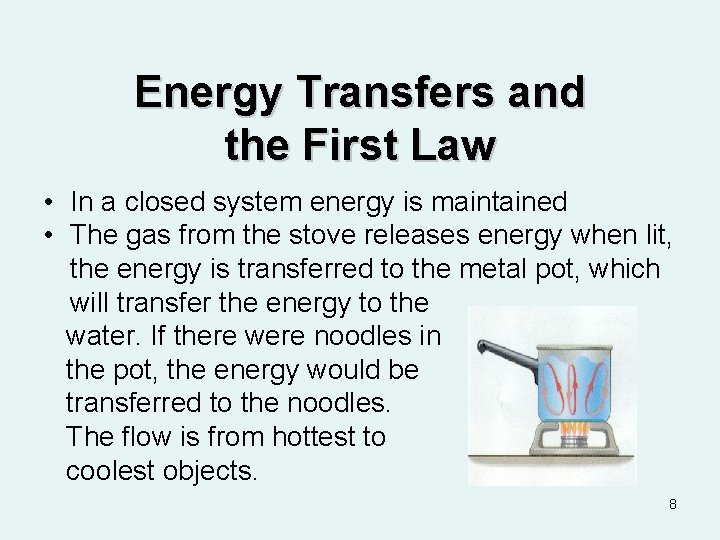
Energy Transfers and the First Law • In a closed system energy is maintained • The gas from the stove releases energy when lit, the energy is transferred to the metal pot, which will transfer the energy to the water. If there were noodles in the pot, the energy would be transferred to the noodles. The flow is from hottest to coolest objects. 8

Energy Transfers and the First Law • Heat is released from the lit gas, the metal pot and the water. Much of this heat energy is transferred to the atmosphere around the stove. The temperature of the room will increase because the molecules in the air are gaining energy and moving faster, which is basically an increase in temperature. • The energy is never lost; it just changes form. 9

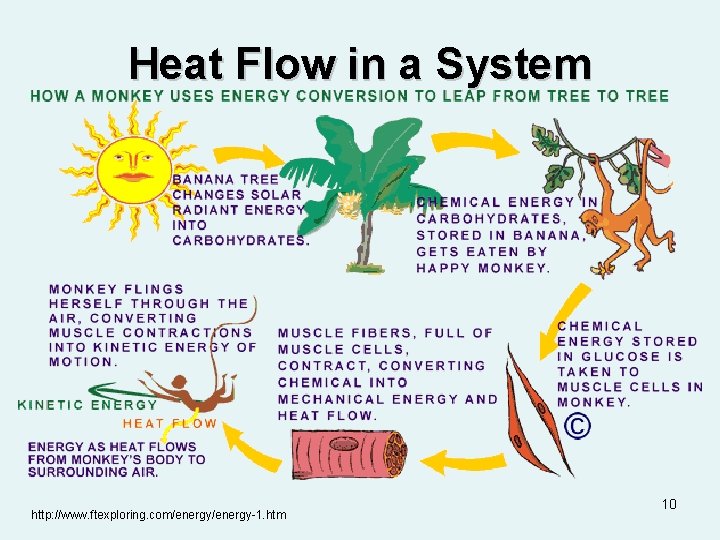
Heat Flow in a System http: //www. ftexploring. com/energy-1. htm 10

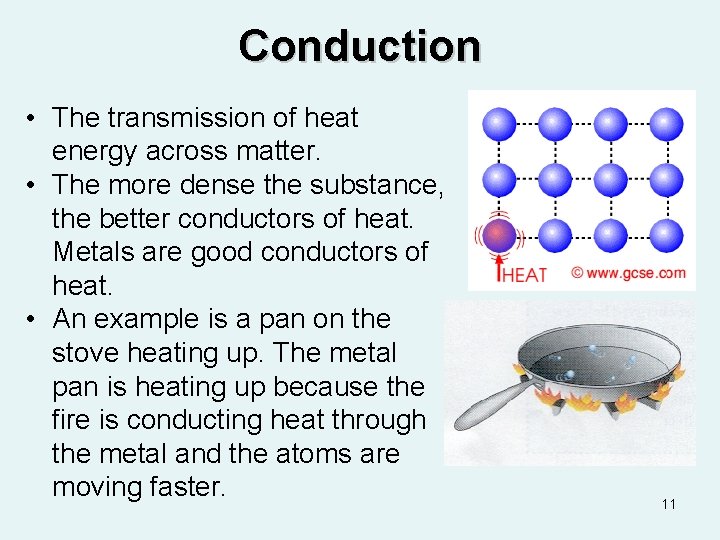
Conduction • The transmission of heat energy across matter. • The more dense the substance, the better conductors of heat. Metals are good conductors of heat. • An example is a pan on the stove heating up. The metal pan is heating up because the fire is conducting heat through the metal and the atoms are moving faster. 11

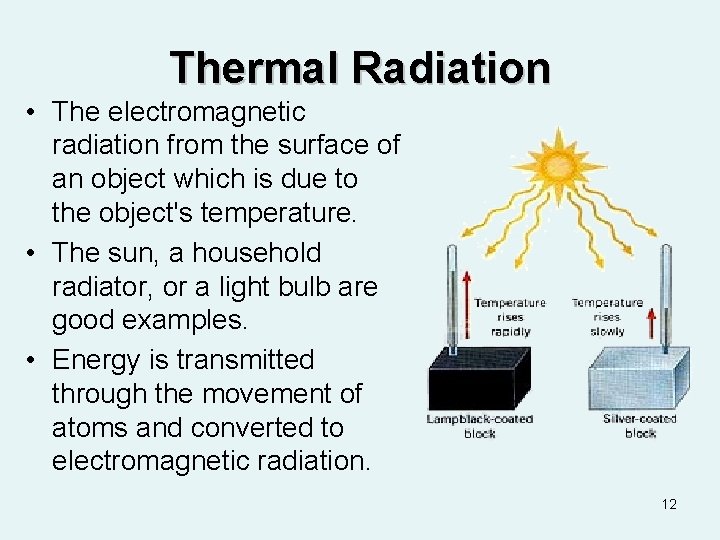
Thermal Radiation • The electromagnetic radiation from the surface of an object which is due to the object's temperature. • The sun, a household radiator, or a light bulb are good examples. • Energy is transmitted through the movement of atoms and converted to electromagnetic radiation. 12

Chemical to Thermal Energy (Heat) • Chemical energy is the energy of the bonds between the atoms of the molecules. • In fuels, like propane, gasoline, or natural gas (methane), heat is generated through an exothermic reaction. The chemical energy in the reactants is greater than in the products. • The release of heat (thermal) energy accounts for the difference between the chemical energy of reactants and the products. The temperature of the environment will tend to increase. 13

Chemical vs. Physical Changes • • Physical Same substance Same chemical properties Affects one or more physical properties of the substance Examples include freezing of water, sanding wood, cutting your hair, mixing, melting Chemical • Substances change into new substances with new properties • Process of change • Examples include sour milk, effervescent tablets, burning of substances, rust 14
 Is a disturbance that transfers energy
Is a disturbance that transfers energy Heat and mass transfer fundamentals and applications
Heat and mass transfer fundamentals and applications Sankey diagram for one bounce of a ball answers
Sankey diagram for one bounce of a ball answers What is heat transfer conduction convection and radiation
What is heat transfer conduction convection and radiation Convection examples
Convection examples Entropy and heat transfer
Entropy and heat transfer Heat and mass transfer cengel 4th edition pdf
Heat and mass transfer cengel 4th edition pdf Fundamental of heat and mass transfer
Fundamental of heat and mass transfer Bulk temperature formula
Bulk temperature formula Single effect evaporator calculations
Single effect evaporator calculations Simultaneous heat and mass transfer
Simultaneous heat and mass transfer Does heat radiation travel in straight lines
Does heat radiation travel in straight lines Heat-mass transfer and geodynamics of the lithosphere:
Heat-mass transfer and geodynamics of the lithosphere: Chapter 7 heat transfer and change of phase
Chapter 7 heat transfer and change of phase Conduction
Conduction