Treatment of Chronic Hepatitis C in HCVHIV Infected








- Slides: 39

Treatment of Chronic Hepatitis C in HCV-HIV Infected Patients J Mallolas Infectious Diseases Service Hospital Clínic Barcelona 1

1. Why to treat HCV in HIV patients ? 2

Why to treat HCV in HIV patients ? 1. Longer survival 2. Faster progression to cirrhosis 3. Higher mortality due to ESLD 4. Higher risk of antiretroviral hepatotoxicity 5. Faster progression of HIV disease 3

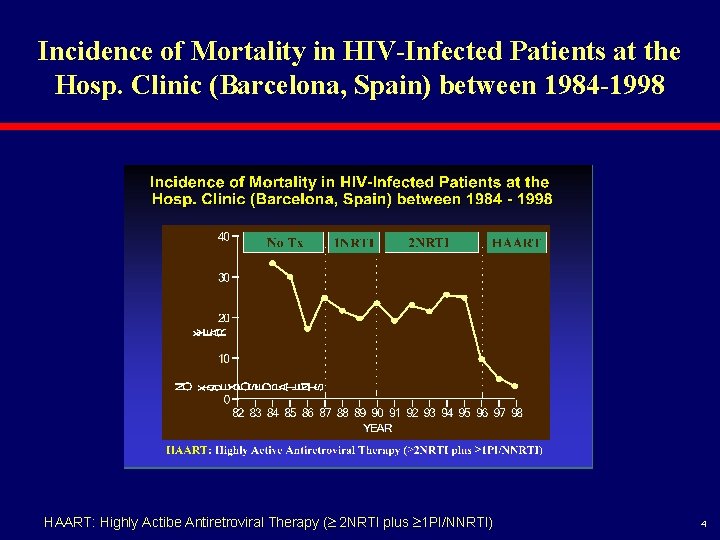
Incidence of Mortality in HIV-Infected Patients at the Hosp. Clinic (Barcelona, Spain) between 1984 -1998 HAART: Highly Actibe Antiretroviral Therapy ( 2 NRTI plus 1 PI/NNRTI) 4

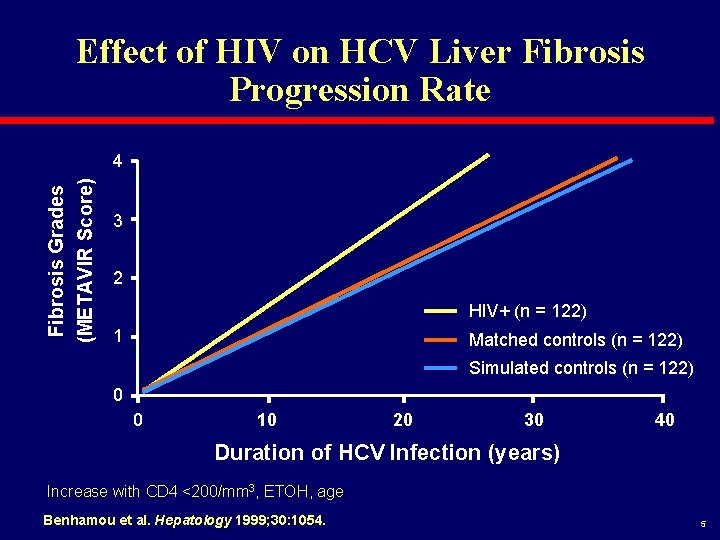
Effect of HIV on HCV Liver Fibrosis Progression Rate Fibrosis Grades (METAVIR Score) 4 3 2 HIV+ (n = 122) 1 Matched controls (n = 122) Simulated controls (n = 122) 0 0 10 20 30 40 Duration of HCV Infection (years) Increase with CD 4 <200/mm 3, ETOH, age Benhamou et al. Hepatology 1999; 30: 1054. 5

Causes of death per year in HIV patients Hospital Clínic. Barcelona 6

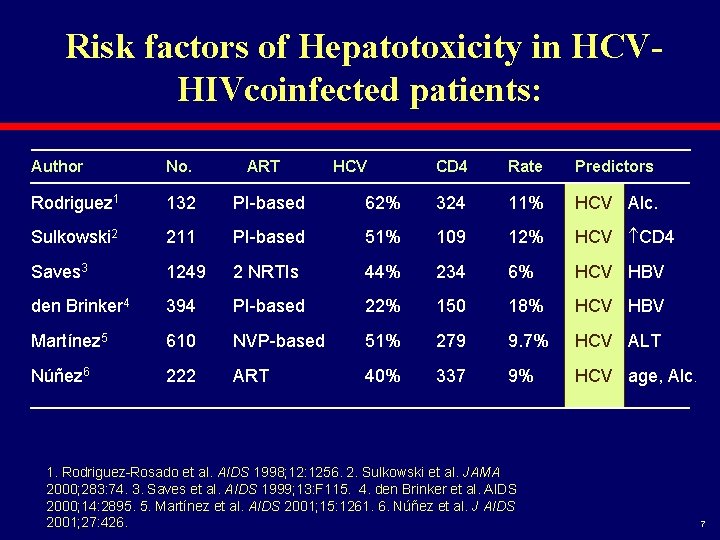
Risk factors of Hepatotoxicity in HCVHIVcoinfected patients: Author No. ART Rodriguez 1 132 PI-based Sulkowski 2 211 Saves 3 HCV CD 4 Rate Predictors 62% 324 11% HCV Alc. PI-based 51% 109 12% HCV CD 4 1249 2 NRTIs 44% 234 6% HCV HBV den Brinker 4 394 PI-based 22% 150 18% HCV HBV Martínez 5 610 NVP-based 51% 279 9. 7% HCV ALT Núñez 6 222 ART 40% 337 9% HCV age, Alc. 1. Rodriguez-Rosado et al. AIDS 1998; 12: 1256. 2. Sulkowski et al. JAMA 2000; 283: 74. 3. Saves et al. AIDS 1999; 13: F 115. 4. den Brinker et al. AIDS 2000; 14: 2895. 5. Martínez et al. AIDS 2001; 15: 1261. 6. Núñez et al. J AIDS 2001; 27: 426. 7

2. How to treat HCV in HIV patients ? 8

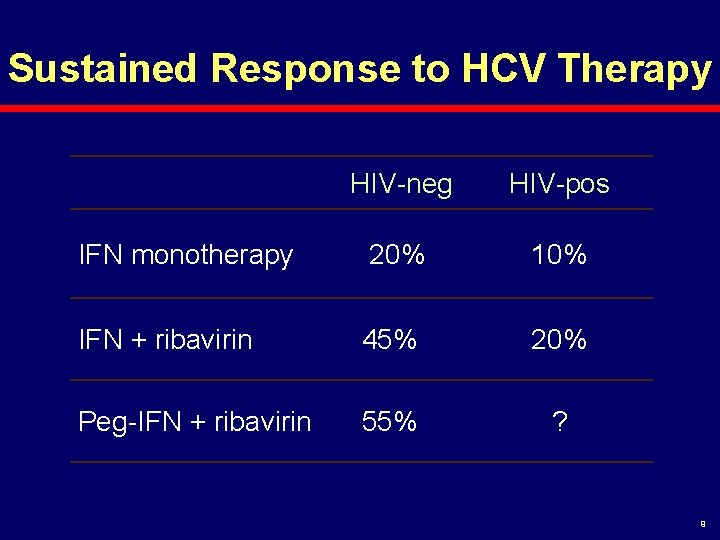
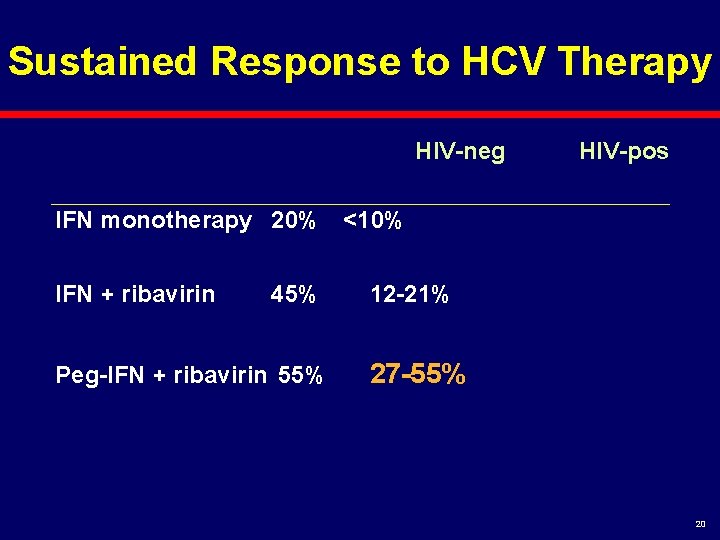
Sustained Response to HCV Therapy HIV-neg HIV-pos IFN monotherapy 20% 10% IFN + ribavirin 45% 20% Peg-IFN + ribavirin 55% ? 9

Peginterferon alfa-2 b plus ribavirin compared with interferon alfa-2 b plus ribavirin for treatment of HIV/HCV co-infected patients AIDS 2004, 18: F 27–F 36 10

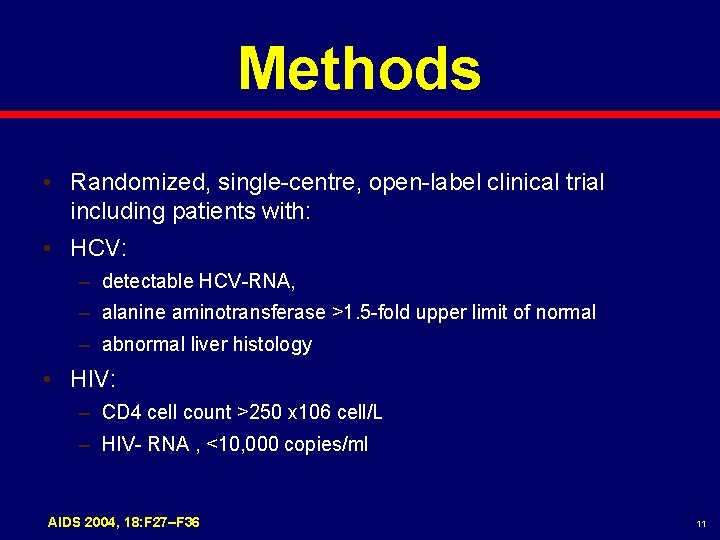
Methods • Randomized, single-centre, open-label clinical trial including patients with: • HCV: – detectable HCV-RNA, – alanine aminotransferase >1. 5 -fold upper limit of normal – abnormal liver histology • HIV: – CD 4 cell count >250 x 106 cell/L – HIV- RNA , <10, 000 copies/ml AIDS 2004, 18: F 27–F 36 11

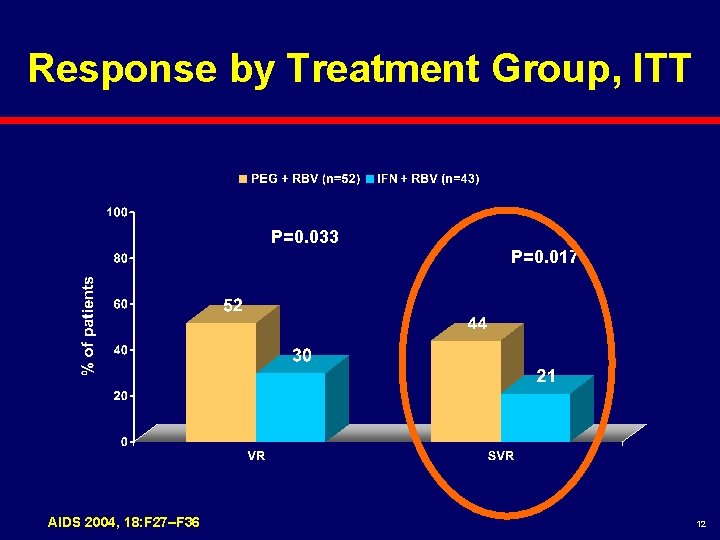
Response by Treatment Group, ITT P=0. 033 AIDS 2004, 18: F 27–F 36 P=0. 017 12

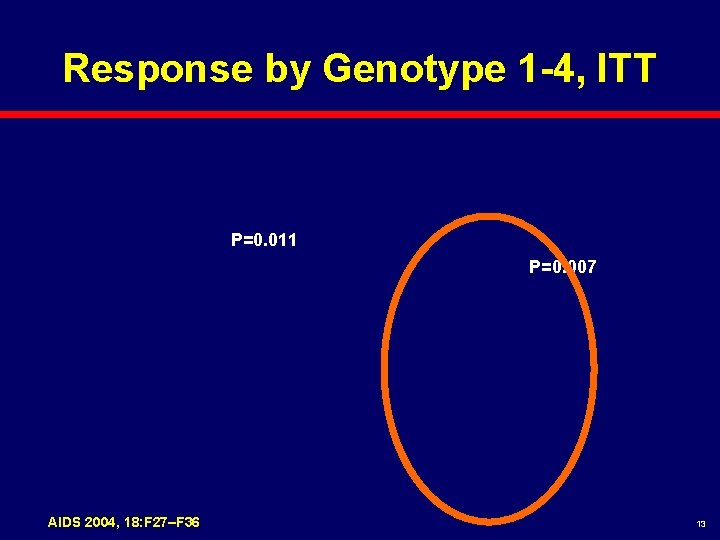
Response by Genotype 1 -4, ITT P=0. 011 P=0. 007 AIDS 2004, 18: F 27–F 36 13

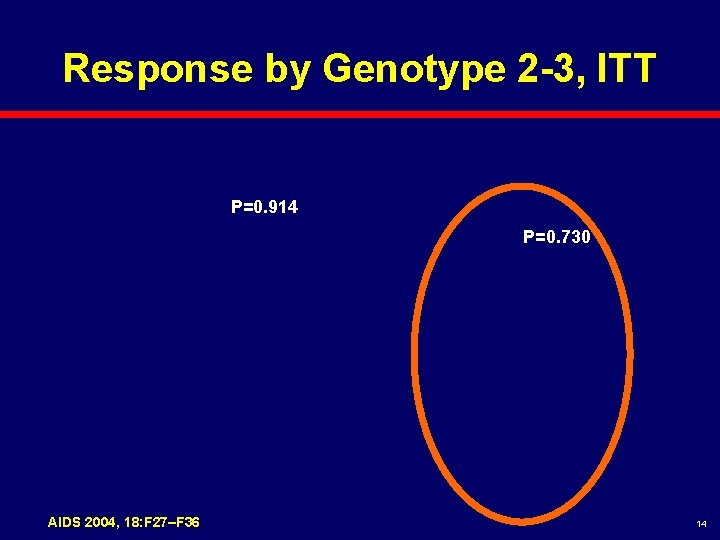
Response by Genotype 2 -3, ITT P=0. 914 P=0. 730 AIDS 2004, 18: F 27–F 36 14

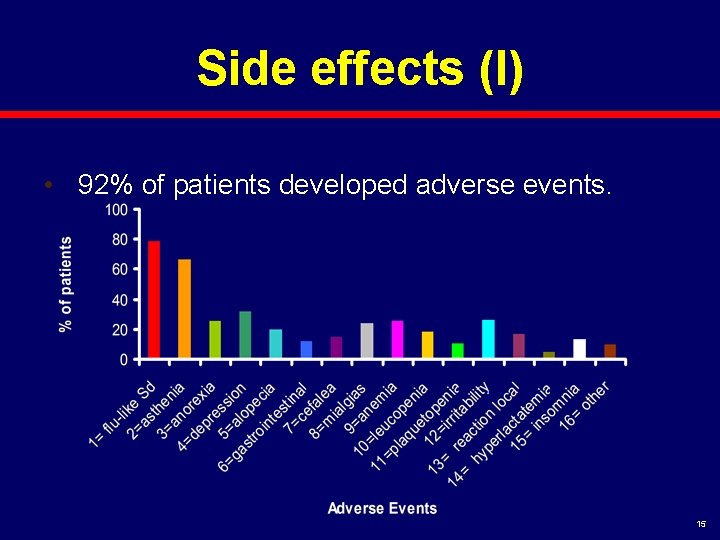
Side effects (I) • 92% of patients developed adverse events. 15

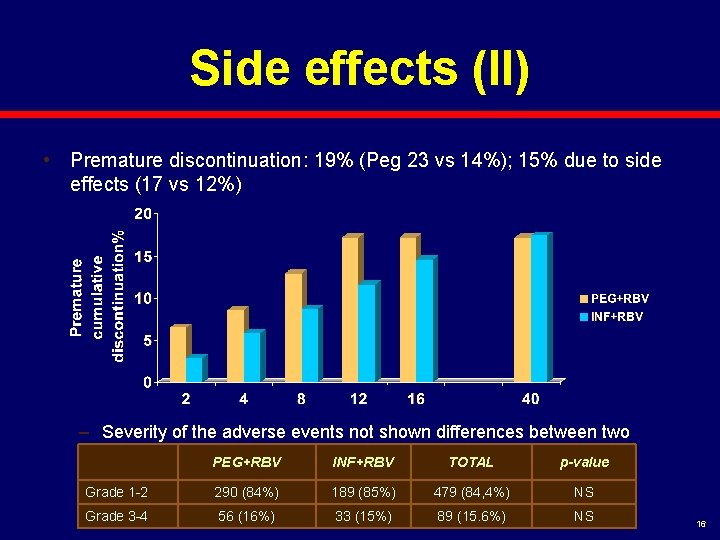
Side effects (II) • Premature discontinuation: 19% (Peg 23 vs 14%); 15% due to side effects (17 vs 12%) – Severity of the adverse events not shown differences between two arms. PEG+RBV INF+RBV TOTAL p-value Grade 1 -2 290 (84%) 189 (85%) 479 (84, 4%) NS Grade 3 -4 56 (16%) 33 (15%) 89 (15. 6%) NS 16

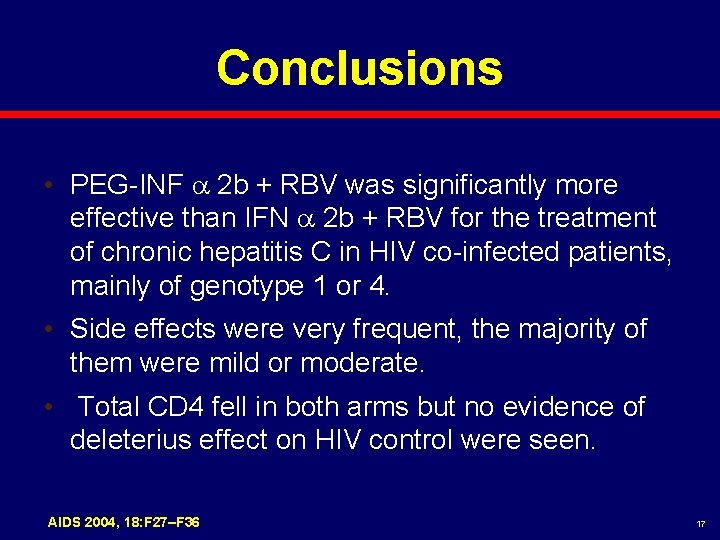
Conclusions • PEG-INF 2 b + RBV was significantly more effective than IFN 2 b + RBV for the treatment of chronic hepatitis C in HIV co-infected patients, mainly of genotype 1 or 4. • Side effects were very frequent, the majority of them were mild or moderate. • Total CD 4 fell in both arms but no evidence of deleterius effect on HIV control were seen. AIDS 2004, 18: F 27–F 36 17

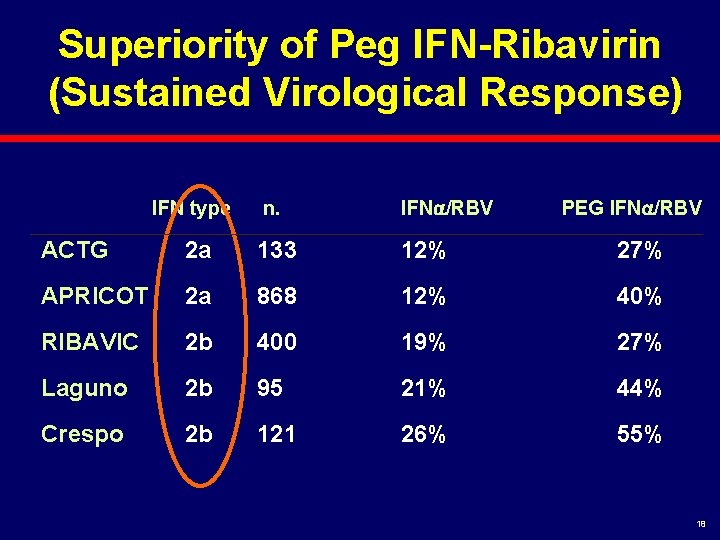
Superiority of Peg IFN-Ribavirin (Sustained Virological Response) IFN type n. IFN /RBV PEG IFN /RBV ACTG 2 a 133 12% 27% APRICOT 2 a 868 12% 40% RIBAVIC 2 b 400 19% 27% Laguno 2 b 95 21% 44% Crespo 2 b 121 26% 55% 18

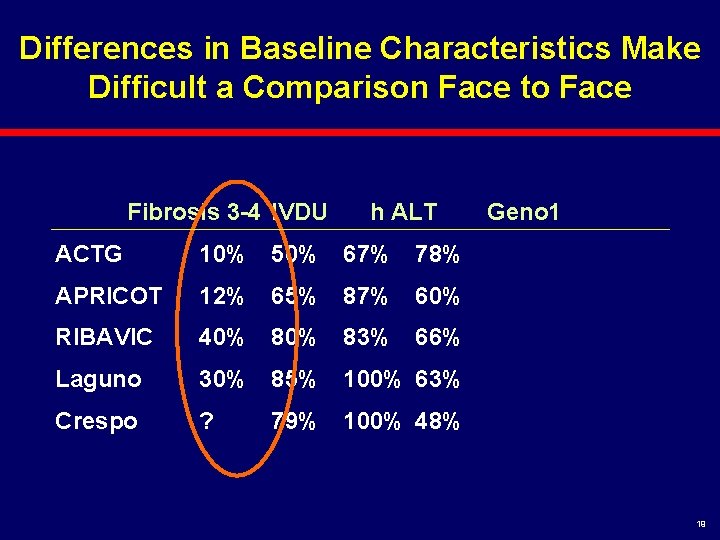
Differences in Baseline Characteristics Make Difficult a Comparison Face to Face Fibrosis 3 -4 IVDU h ALT ACTG 10% 50% 67% 78% APRICOT 12% 65% 87% 60% RIBAVIC 40% 83% 66% Laguno 30% 85% 100% 63% Crespo ? Geno 1 79% 100% 48% 19

Sustained Response to HCV Therapy HIV-neg HIV-pos IFN monotherapy 20% <10% IFN + ribavirin 45% Peg-IFN + ribavirin 55% 12 -21% 27 -55% 20

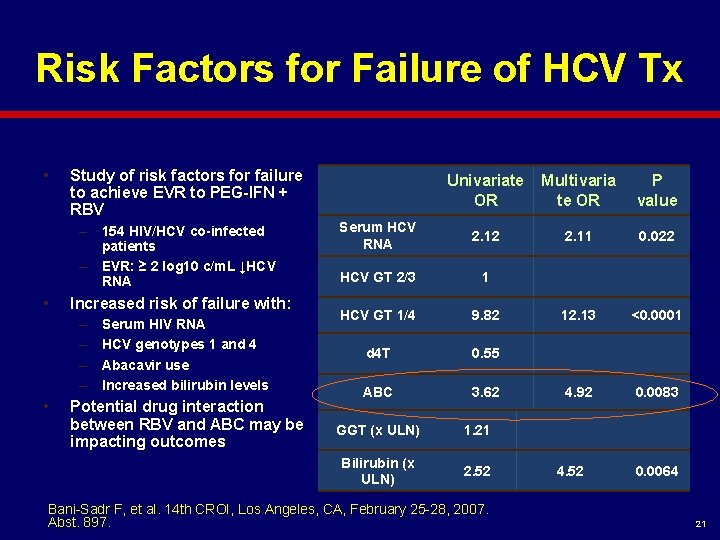
Risk Factors for Failure of HCV Tx • Study of risk factors for failure to achieve EVR to PEG-IFN + RBV – 154 HIV/HCV co-infected patients – EVR: ≥ 2 log 10 c/m. L ↓HCV RNA • Increased risk of failure with: – – • Serum HIV RNA HCV genotypes 1 and 4 Abacavir use Increased bilirubin levels Potential drug interaction between RBV and ABC may be impacting outcomes Univariate OR Multivaria te OR P value Serum HCV RNA 2. 12 2. 11 0. 022 HCV GT 2/3 1 HCV GT 1/4 9. 82 12. 13 <0. 0001 d 4 T 0. 55 ABC 3. 62 4. 92 0. 0083 GGT (x ULN) 1. 21 Bilirubin (x ULN) 2. 52 Bani-Sadr F, et al. 14 th CROI, Los Angeles, CA, February 25 -28, 2007. Abst. 897. 4. 52 0. 0064 21

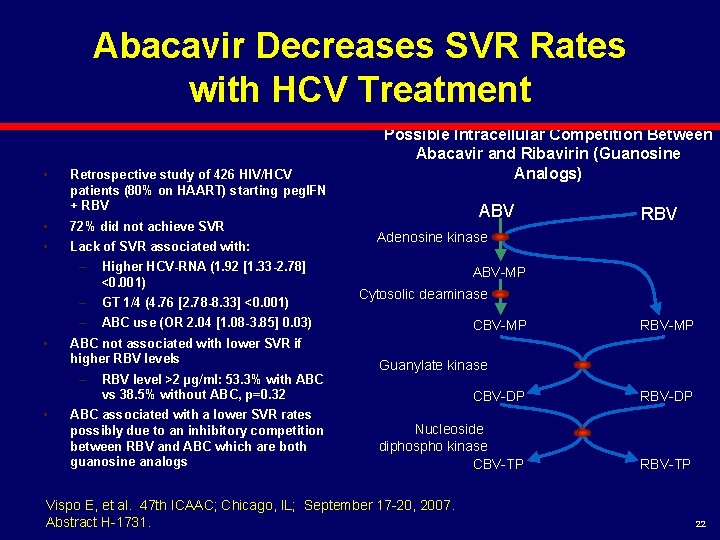
Abacavir Decreases SVR Rates with HCV Treatment • Retrospective study of 426 HIV/HCV patients (80% on HAART) starting peg. IFN + RBV • 72% did not achieve SVR • Lack of SVR associated with: • – Higher HCV-RNA (1. 92 [1. 33 -2. 78] <0. 001) – GT 1/4 (4. 76 [2. 78 -8. 33] <0. 001) – ABC use (OR 2. 04 [1. 08 -3. 85] 0. 03) ABC not associated with lower SVR if higher RBV levels – • RBV level >2 µg/ml: 53. 3% with ABC vs 38. 5% without ABC, p=0. 32 ABC associated with a lower SVR rates possibly due to an inhibitory competition between RBV and ABC which are both guanosine analogs Possible Intracellular Competition Between Abacavir and Ribavirin (Guanosine Analogs) ABV RBV Adenosine kinase ABV-MP Cytosolic deaminase CBV-MP RBV-MP Guanylate kinase CBV-DP RBV-DP Nucleoside diphospho kinase CBV-TP RBV-TP Vispo E, et al. 47 th ICAAC; Chicago, IL; September 17 -20, 2007. Abstract H-1731. 22

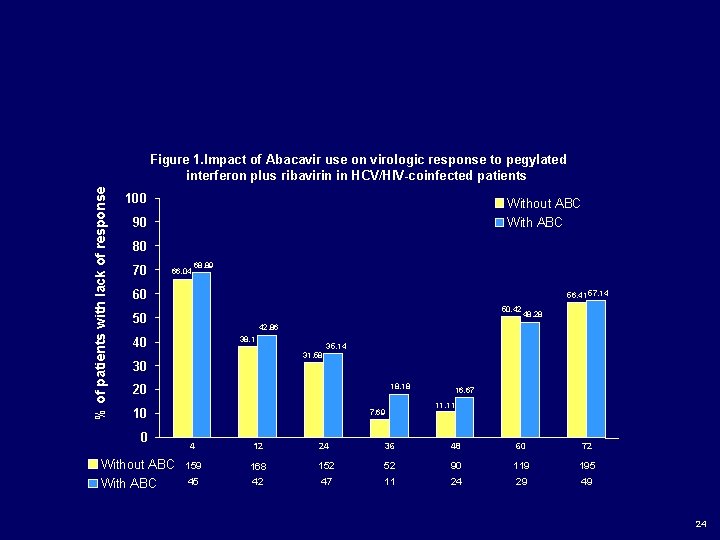
Abacavir does not influence the rate of sustained virological response in HIV-HCV co-infected patients treated with pegylated interferon and weight adjusted ribavirin Authors: Laufer N 1, Laguno M 1, Perez I 2, Cifuentes C 3, Murillas J 4, Vidal F 5, Bonet L 4, Veloso S 5, Gatell JM 1; Mallolas J 1. *** Antiviral Therapy (submitted for publication) 23

% of patients with lack of response Figure 1. Impact of Abacavir use on virologic response to pegylated interferon plus ribavirin in HCV/HIV-coinfected patients 100 Without ABC With ABC 90 80 70 66, 04 68, 89 60 56, 41 57, 14 50, 42 50 38, 1 40 35, 14 31, 58 30 18, 18 20 10 0 Without ABC With ABC 48, 28 42, 86 7, 69 16, 67 11, 11 4 12 24 36 48 60 72 159 168 152 52 90 119 195 45 42 47 11 24 29 49 24

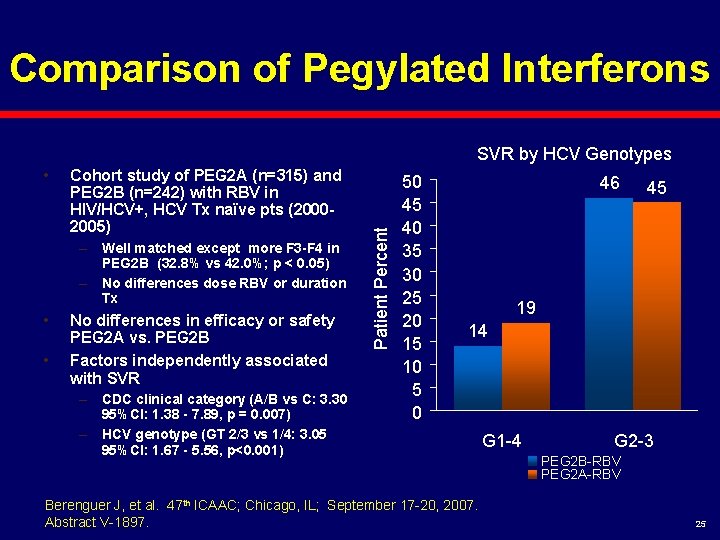
Comparison of Pegylated Interferons • Cohort study of PEG 2 A (n=315) and PEG 2 B (n=242) with RBV in HIV/HCV+, HCV Tx naïve pts (20002005) – Well matched except more F 3 -F 4 in PEG 2 B (32. 8% vs 42. 0%; p < 0. 05) – No differences dose RBV or duration Tx • • No differences in efficacy or safety PEG 2 A vs. PEG 2 B Factors independently associated with SVR – CDC clinical category (A/B vs C: 3. 30 95%CI: 1. 38 - 7. 89, p = 0. 007) – HCV genotype (GT 2/3 vs 1/4: 3. 05 95%CI: 1. 67 - 5. 56, p<0. 001) Patient Percent SVR by HCV Genotypes 50 45 40 35 30 25 20 15 10 5 0 46 45 19 14 Berenguer J, et al. 47 th ICAAC; Chicago, IL; September 17 -20, 2007. Abstract V-1897. G 1 -4 G 2 -3 PEG 2 B-RBV PEG 2 A-RBV 25

Poster: 1018 b A randomized trial to compare the efficacy and safety of PEG-interferon (PEG) alfa-2 b plus ribavirin (RBV) vs PEG alfa-2 a plus RBV for treatment of chronic hepatitis C in HIV co-infected patients. Laguno M 1, Cifuentes C 2, Murillas J 3, Vidal F 4, Bonet L 3, Veloso S 4, Tural C 5, Perez I 1, Gatell JM 1, Mallolas J 1. 1 Hospital Clínic. Barcelona. Spain. 2 Hospital Son Llàtzer. Mallorca. Spain. 3 Hospital Son Dureta. Mallorca. Spain. 4 Hospital Joan XXIII. Tarragona. Spain. 5 Hospital Germans Trias i Pujol. Badalona. Spain. E-mail: mallolas@clinic. ub. es Tel. +34 -93 -2275574 FAX. + 34 -93 -4514438 CROI-2008. Boston. USA 26

METHODS • Prospective, randomized, multi-centre, open-label clinical trial • Inclusion criteria: – – Detectable HCV-RNA Alanine aminotransferase >1. 5 -fold upper normal limit Abnormal liver histology CD 4 counts >250 cells/mm 3 and HIV-RNA <50000 copies/m. L. • Treatment arms: PEG 2 b (80 -150 µg/wk adjusted to body weight) or PEG 2 a (180µg/wk) + RBV (800 -1200 mg/d adjusted to body weight) in both arms • Duration of treatment: 48 weeks. 27

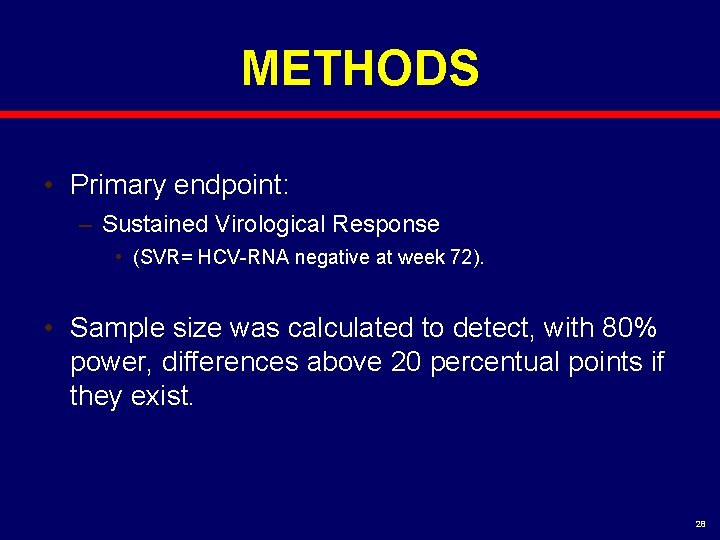
METHODS • Primary endpoint: – Sustained Virological Response • (SVR= HCV-RNA negative at week 72). • Sample size was calculated to detect, with 80% power, differences above 20 percentual points if they exist. 28

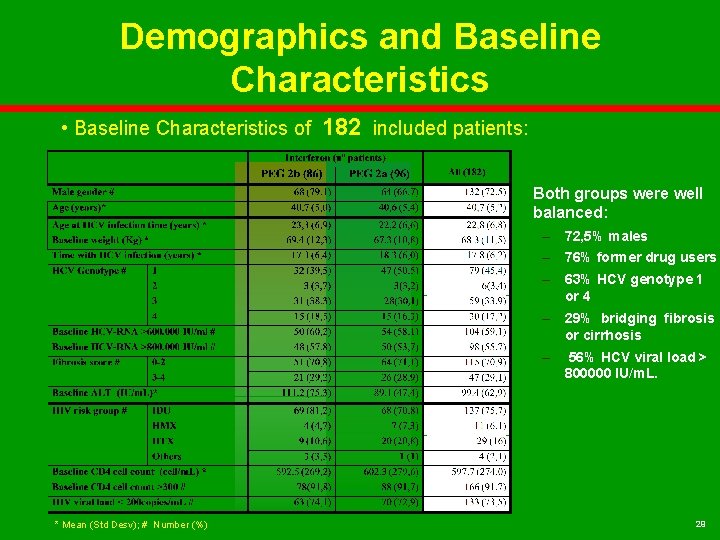
Demographics and Baseline Characteristics • Baseline Characteristics of 182 included patients: Both groups were well balanced: – 72, 5% males – 76% former drug users – 63% HCV genotype 1 or 4 – 29% bridging fibrosis or cirrhosis – * Mean (Std Desv); # Number (%) 56% HCV viral load > 800000 IU/m. L. 29

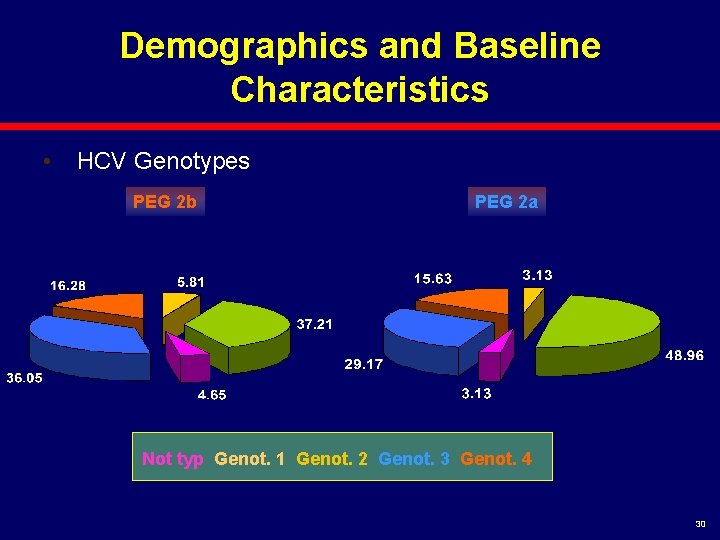
Demographics and Baseline Characteristics • HCV Genotypes PEG 2 b PEG 2 a Not typ Genot. 1 Genot. 2 Genot. 3 Genot. 4 30

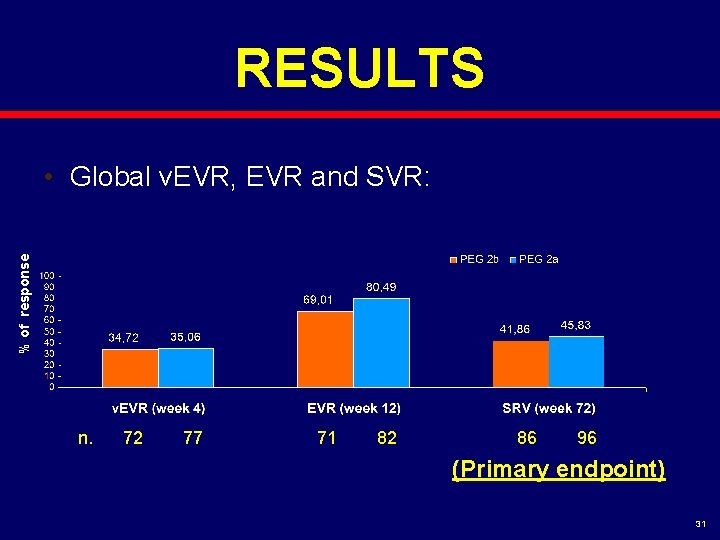
RESULTS % of response • Global v. EVR, EVR and SVR: n. 72 77 71 82 86 96 (Primary endpoint) 31

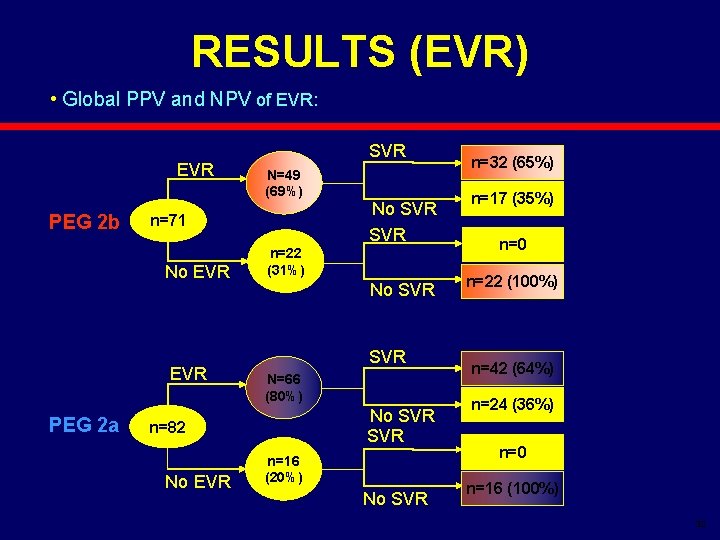
RESULTS (EVR) • Global PPV and NPV of EVR: EVR PEG 2 b N=49 (69%) n=71 No EVR PEG 2 a SVR n=22 (31%) No SVR N=66 (80%) No SVR n=82 No EVR No SVR n=16 (20%) No SVR n=32 (65%) n=17 (35%) n=0 n=22 (100%) n=42 (64%) n=24 (36%) n=0 n=16 (100%) 32

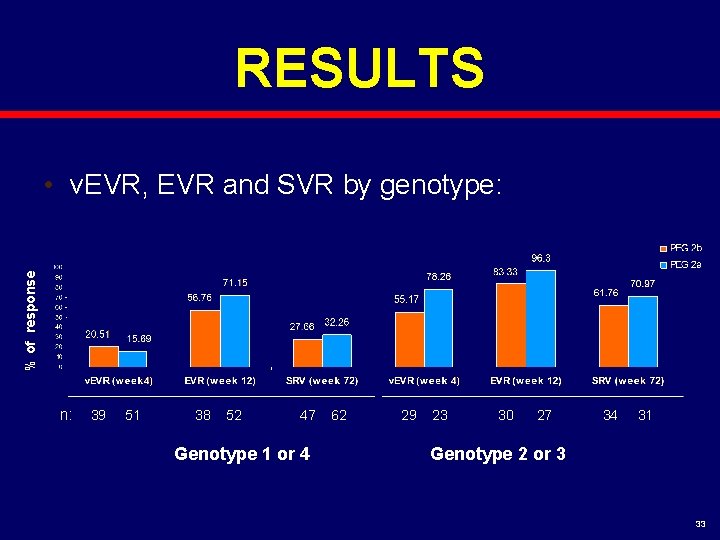
RESULTS % of response • v. EVR, EVR and SVR by genotype: n: 39 51 38 52 47 Genotype 1 or 4 62 29 23 30 27 34 31 Genotype 2 or 3 33

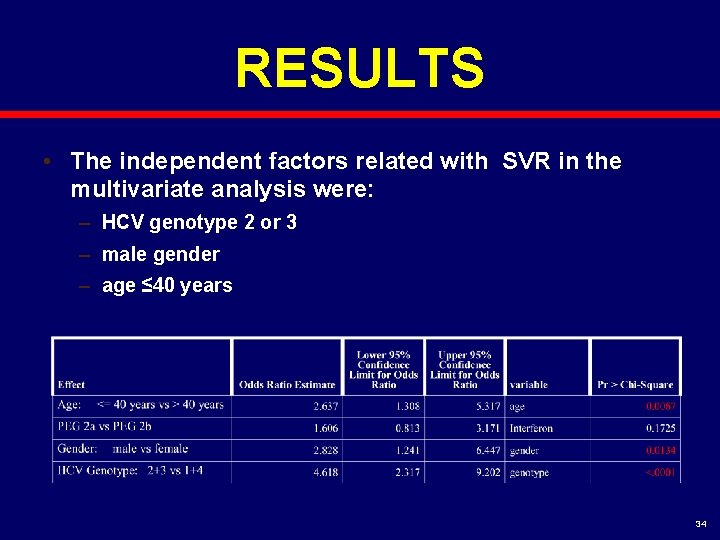
RESULTS • The independent factors related with SVR in the multivariate analysis were: – HCV genotype 2 or 3 – male gender – age ≤ 40 years 34

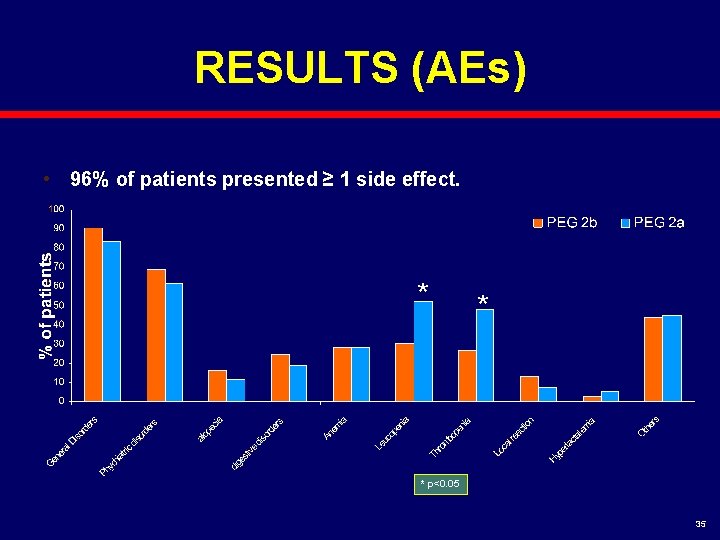
RESULTS (AEs) • 96% of patients presented ≥ 1 side effect. * * * p<0. 05 35

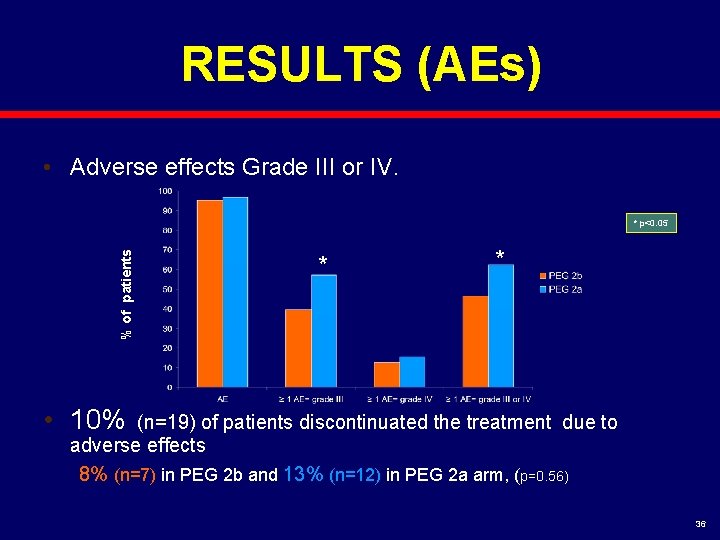
RESULTS (AEs) • Adverse effects Grade III or IV. % of patients * p<0. 05 * * • 10% (n=19) of patients discontinuated the treatment due to adverse effects 8% (n=7) in PEG 2 b and 13% (n=12) in PEG 2 a arm, (p=0. 56) 36

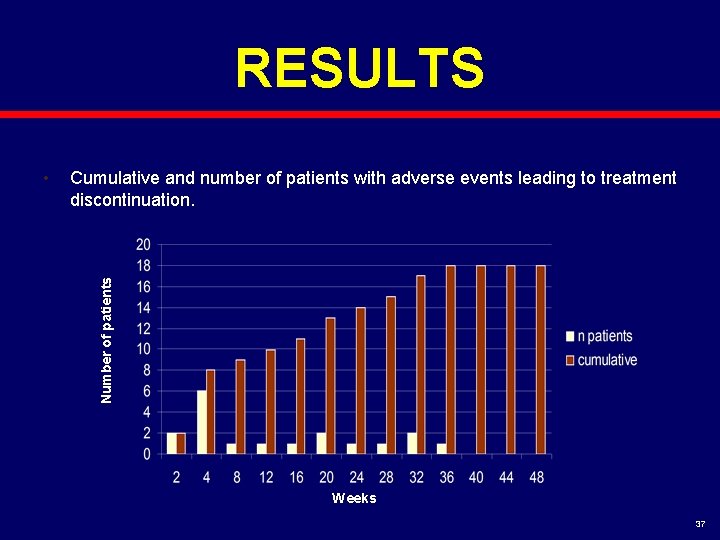
RESULTS Cumulative and number of patients with adverse events leading to treatment discontinuation. Number of patients • Weeks 37

CONCLUSION • In HIV infected patients, treatment of chronic HCV with RBV plus PEG 2 b or PEG 2 a had no statistically significant differences in tolerance and efficacy. 38

Acknowledments Infections Diseases Service: Laguno M Murillas J León A Blanco JL García-Gasalla M Martínez E Milinkovic A Loncá M Callau P Miró JM Poal M Rodriguez A Casadesus C García F Gatell JM Mallolas J *** Radiology Service: Bianchi L Vilana R Gilabert R García-Criado A Bargalló X Hepatology Service: Sánchez-Tapias JM Pathology Service: Miquel R Biostatistics: de Lazzari E Pérez I Phyquiatry Service: Blanch J To the Patients 39
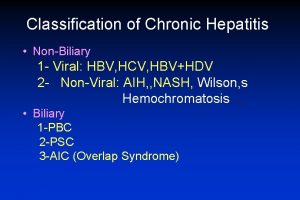
 Classification of chronic hepatitis
Classification of chronic hepatitis Hepatitis causes
Hepatitis causes Chronic hepatitis
Chronic hepatitis Hep b mode of transmission
Hep b mode of transmission Pbc vs psc
Pbc vs psc Hepatitis c
Hepatitis c Hepatitis a treatment
Hepatitis a treatment Chronic myeloid leukemia treatment
Chronic myeloid leukemia treatment Rubin's postpartum phases
Rubin's postpartum phases Cavity varnish example
Cavity varnish example Lochia smell
Lochia smell What does infected lochia look like
What does infected lochia look like Malaria parasites under microscope
Malaria parasites under microscope What does infected lochia look like
What does infected lochia look like Symptoms of gonorrhea
Symptoms of gonorrhea Hepatitis b markers interpretation
Hepatitis b markers interpretation Health education on hepatitis ppt
Health education on hepatitis ppt Neonatal jaundice
Neonatal jaundice Infectious canine hepatitis in dogs
Infectious canine hepatitis in dogs Hep b vaccine schedule for adults
Hep b vaccine schedule for adults Klasifikasi hepatitis a
Klasifikasi hepatitis a Alcoholic hepatitis
Alcoholic hepatitis Hepatitis c symptoms
Hepatitis c symptoms Hepatitis b is a silent killer
Hepatitis b is a silent killer Std refers to *
Std refers to * Hepatitis lupica
Hepatitis lupica Chronic pancreatitis nursing care plan
Chronic pancreatitis nursing care plan Hepatitis b panel
Hepatitis b panel Hepatitis c medications
Hepatitis c medications Hepatitis e
Hepatitis e Michael le md
Michael le md Types of cirrhosis
Types of cirrhosis Hepatitis types chart
Hepatitis types chart Dosis de lactulosa en encefalopatia hepatica
Dosis de lactulosa en encefalopatia hepatica Esquema basico de vacunacion
Esquema basico de vacunacion Ano ang pagkakaiba ng hepatitis a at b
Ano ang pagkakaiba ng hepatitis a at b Score simplificado hepatitis autoinmune
Score simplificado hepatitis autoinmune Bare area liver
Bare area liver Window period of hepatitis b
Window period of hepatitis b Hepatitis b cronica
Hepatitis b cronica