Transition Metals and Coordination Chemistry Chapter 19 Email

![Transition Metals and Coordination Chemistry – Ch. 19 14. Solutions of [Co(NH 3)6]3+ , Transition Metals and Coordination Chemistry – Ch. 19 14. Solutions of [Co(NH 3)6]3+ ,](https://slidetodoc.com/presentation_image_h/1cc8a71a78acbc2aa57f007255d7aeb2/image-18.jpg)
![Transition Metals and Coordination Chemistry – Ch. 19 13. Solutions of [Co(NH 3)6]3+ , Transition Metals and Coordination Chemistry – Ch. 19 13. Solutions of [Co(NH 3)6]3+ ,](https://slidetodoc.com/presentation_image_h/1cc8a71a78acbc2aa57f007255d7aeb2/image-32.jpg)
- Slides: 33

Transition Metals and Coordination Chemistry Chapter 19 E-mail: benzene 4 president@gmail. com Web-site: http: //clas. sa. ucsb. edu/staff/terri/

Transition Metals and Coordination Chemistry – Ch. 19 1. Which of the following substances can NOT act as a ligand? a. H 2 O b. OH– c. CO d. NH 4+ e. Br–

Transition Metals and Coordination Chemistry – Ch. 19 2. Which of the following can act as a chelate? Circle all that apply. a. NH 2 CH 2 NH 2 b. C 2 O 42– c. NH 2 CH 2 NHCH 2 NH 2 d. SCN–

Transition Metals and Coordination Chemistry – Ch. 19

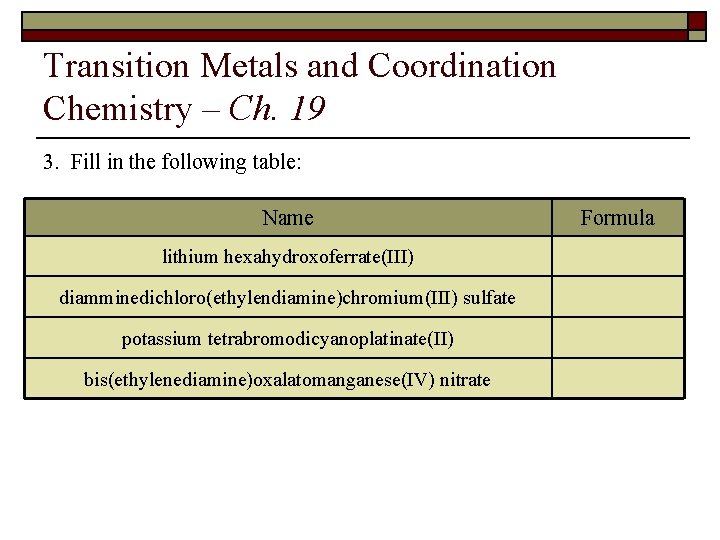
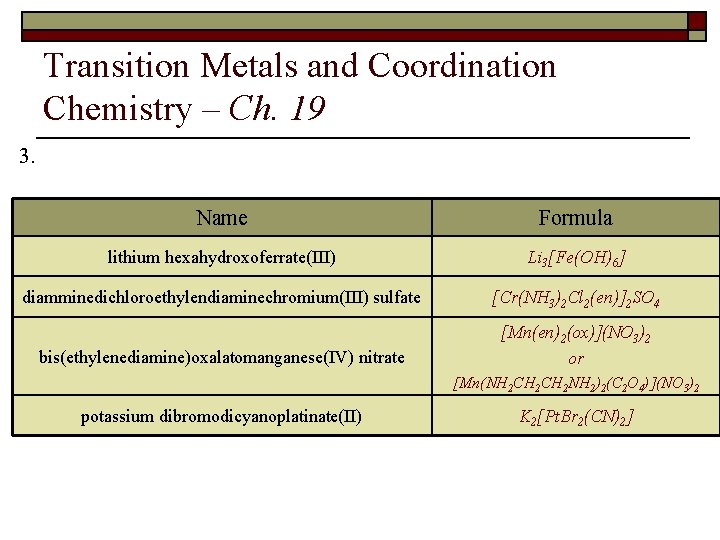
Transition Metals and Coordination Chemistry – Ch. 19 3. Fill in the following table: Name lithium hexahydroxoferrate(III) diamminedichloro(ethylendiamine)chromium(III) sulfate potassium tetrabromodicyanoplatinate(II) bis(ethylenediamine)oxalatomanganese(IV) nitrate Formula

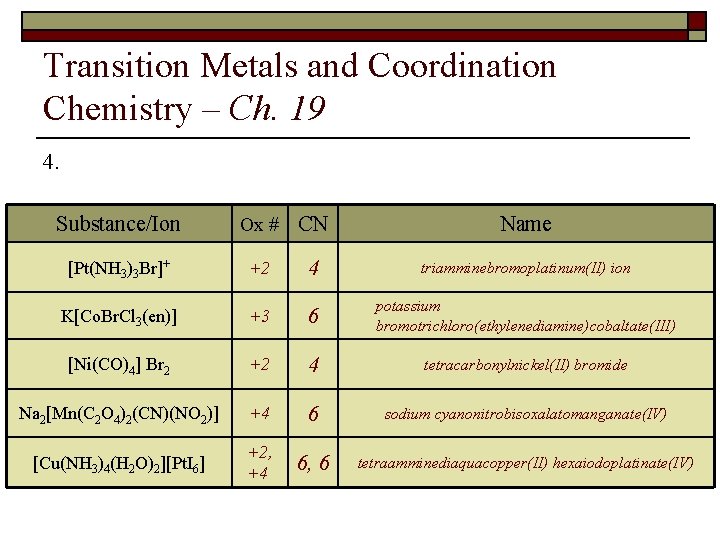
Transition Metals and Coordination Chemistry – Ch. 19 4. Fill in the following table: Substance/Ion [Pt(NH 3)3 Br]+ K[Co(en)Br. Cl 3] [Ni(CO)4] Br 2 Na[Mn(C 2 O 4)2(CN)2] [Cu(NH 3)4(H 2 O)2][Pt. I 6] Ox # Coord. # Name

Transition Metals and Coordination Chemistry – Ch. 19 5. Which of the following can NOT form linkage isomers? a. SCN– b. NO 2– c. NO 3– d. all can form linkage isomers

Transition Metals and Coordination Chemistry – Ch. 19 6. Rank the following 0. 1 M aqueous solutions by their conductivity in water. I. [Cr(H 2 O)6]Cl 3 II. [Cr(H 2 O)4 Cl 2]Cl • (H 2 O)2 III. [Cr(H 2 O)5 Cl]Cl 2 • H 2 O What type of isomers are illustrated above?

Transition Metals and Coordination Chemistry – Ch. 19 7. Which of the following substances can have geometric isomers? a. [Co(NH 3)5 Cl]SO 4 b. [Co(NH 3)6]Cl 3 c. Li[Co(NH 3)3 Cl 3] d. K[Co(NH 3)2 Cl 4] e. Na 3[Co. Cl 6]

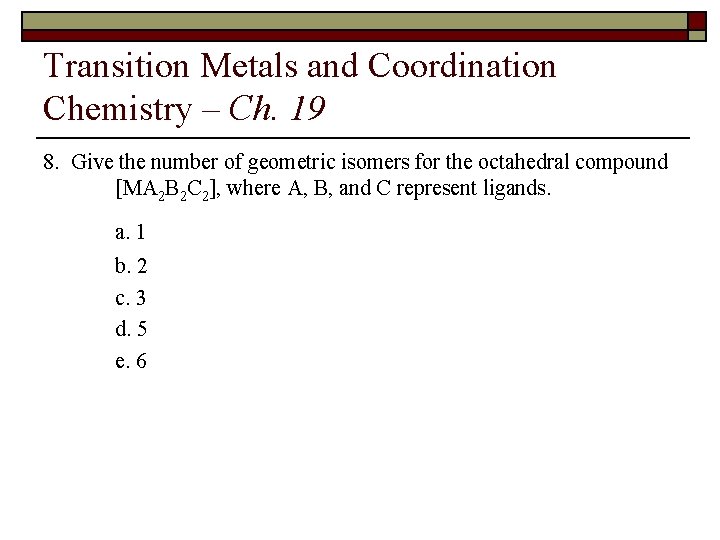
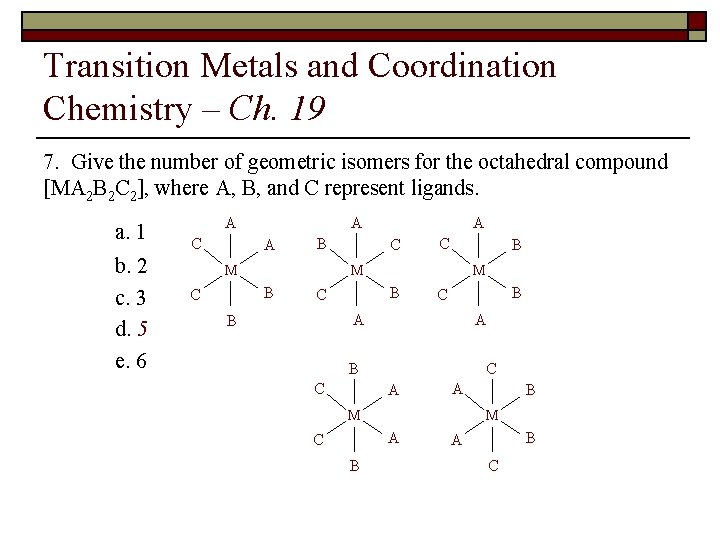
Transition Metals and Coordination Chemistry – Ch. 19 8. Give the number of geometric isomers for the octahedral compound [MA 2 B 2 C 2], where A, B, and C represent ligands. a. 1 b. 2 c. 3 d. 5 e. 6

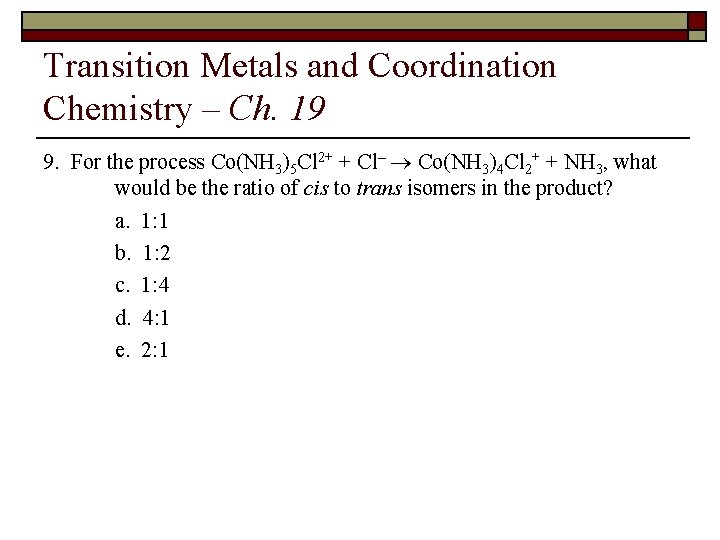
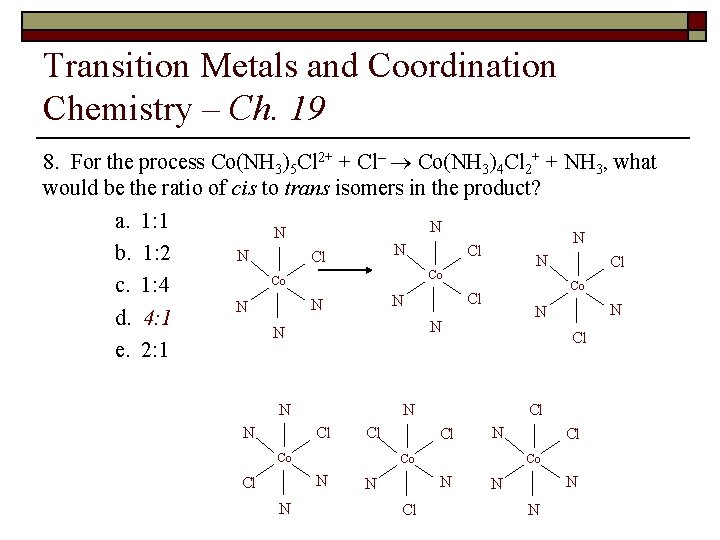
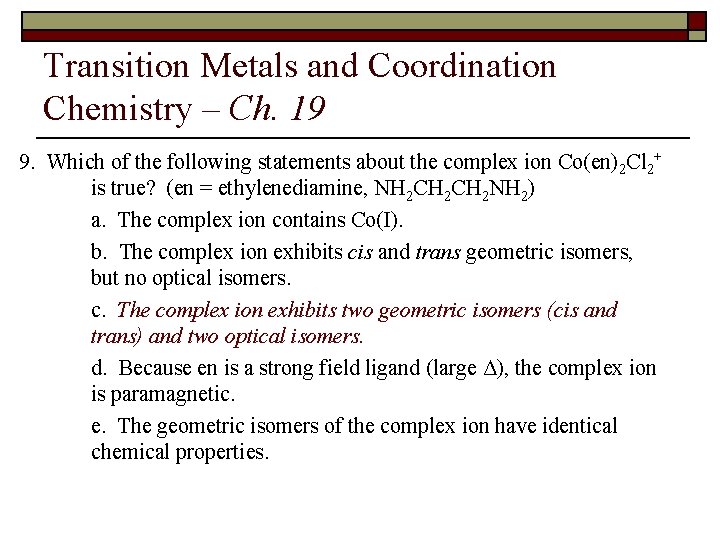
Transition Metals and Coordination Chemistry – Ch. 19 9. For the process Co(NH 3)5 Cl 2+ + Cl– ® Co(NH 3)4 Cl 2+ + NH 3, what would be the ratio of cis to trans isomers in the product? a. 1: 1 b. 1: 2 c. 1: 4 d. 4: 1 e. 2: 1

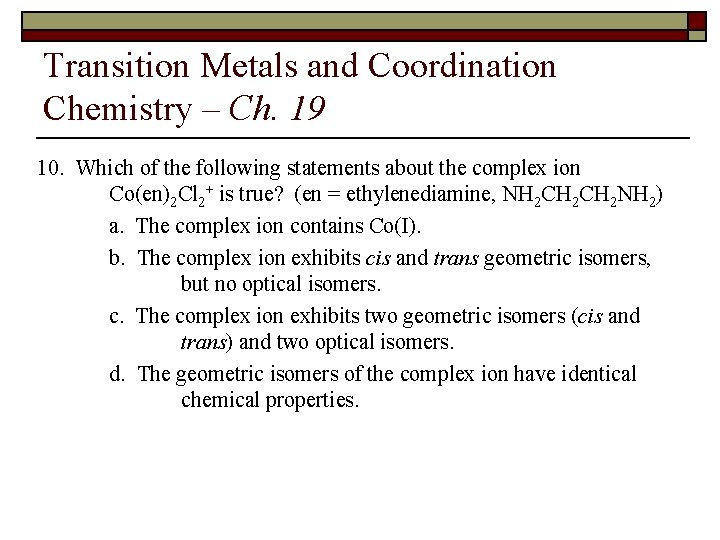
Transition Metals and Coordination Chemistry – Ch. 19 10. Which of the following statements about the complex ion Co(en)2 Cl 2+ is true? (en = ethylenediamine, NH 2 CH 2 NH 2) a. The complex ion contains Co(I). b. The complex ion exhibits cis and trans geometric isomers, but no optical isomers. c. The complex ion exhibits two geometric isomers (cis and trans) and two optical isomers. d. The geometric isomers of the complex ion have identical chemical properties.

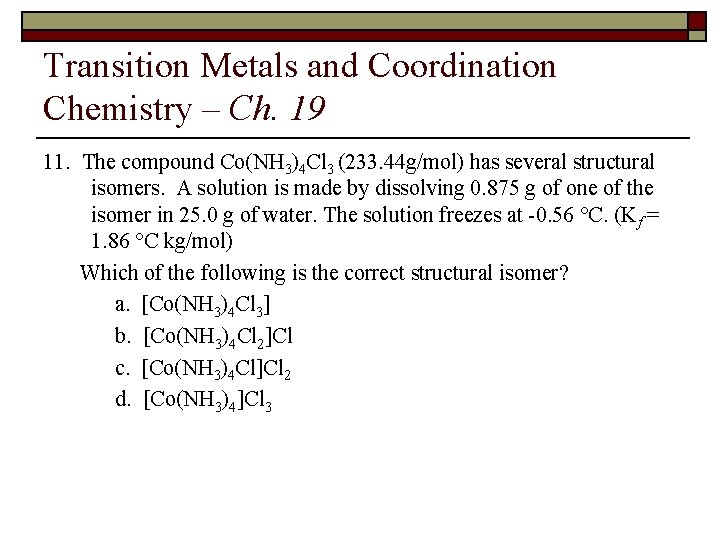
Transition Metals and Coordination Chemistry – Ch. 19 11. The compound Co(NH 3)4 Cl 3 (233. 44 g/mol) has several structural isomers. A solution is made by dissolving 0. 875 g of one of the isomer in 25. 0 g of water. The solution freezes at -0. 56 °C. (Kf = 1. 86 °C kg/mol) Which of the following is the correct structural isomer? a. [Co(NH 3)4 Cl 3] b. [Co(NH 3)4 Cl 2]Cl c. [Co(NH 3)4 Cl]Cl 2 d. [Co(NH 3)4]Cl 3

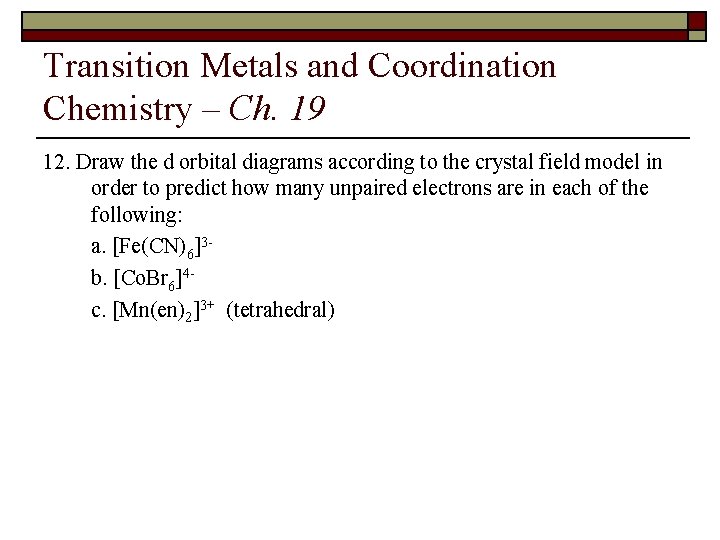
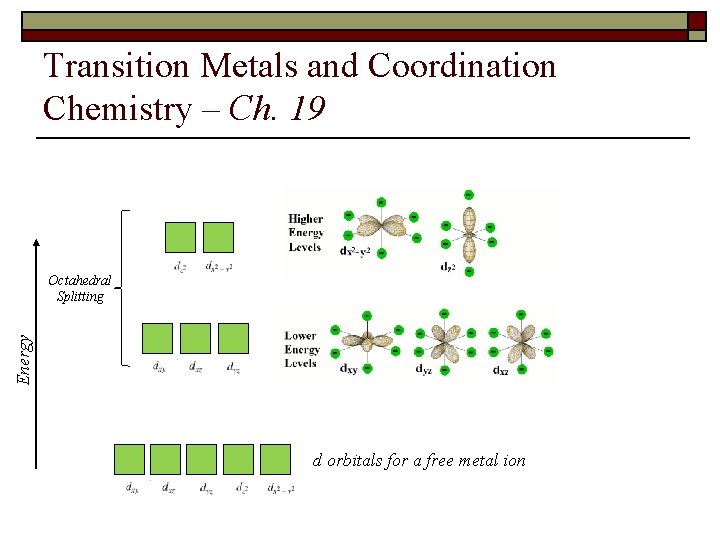
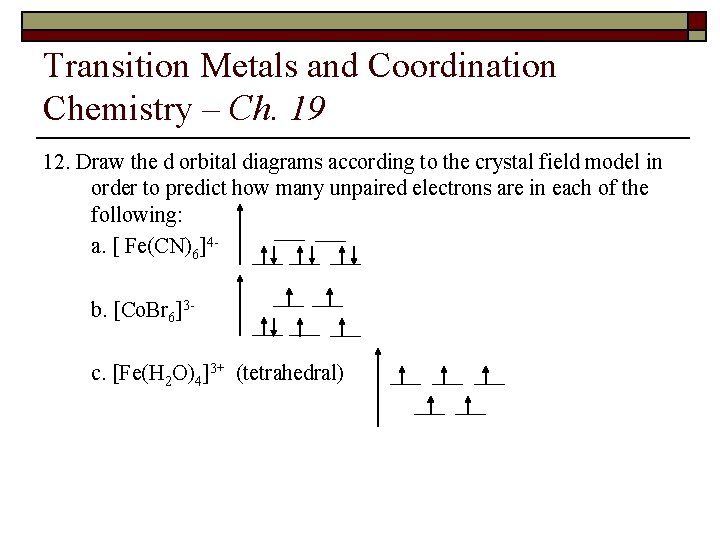
Transition Metals and Coordination Chemistry – Ch. 19 12. Draw the d orbital diagrams according to the crystal field model in order to predict how many unpaired electrons are in each of the following: a. [Fe(CN)6]3 b. [Co. Br 6]4 c. [Mn(en)2]3+ (tetrahedral)

Transition Metals and Coordination Chemistry – Ch. 19 Energy Octahedral Splitting d orbitals for a free metal ion

Transition Metals and Coordination Chemistry – Ch. 19 Energy Tetrahedral Splitting d orbitals for a free metal ion

Transition Metals and Coordination Chemistry – Ch. 19 13. An orange complex and a violet complex were both found to have ferric ion as their central metal; which one absorbs light with a longer wavelength?
![Transition Metals and Coordination Chemistry Ch 19 14 Solutions of CoNH 363 Transition Metals and Coordination Chemistry – Ch. 19 14. Solutions of [Co(NH 3)6]3+ ,](https://slidetodoc.com/presentation_image_h/1cc8a71a78acbc2aa57f007255d7aeb2/image-18.jpg)
Transition Metals and Coordination Chemistry – Ch. 19 14. Solutions of [Co(NH 3)6]3+ , [Co(NO 2)6]3 -and [Co. F 6]3 - are colored. The solutions were red, yellow and blue. Match the solution to the color.

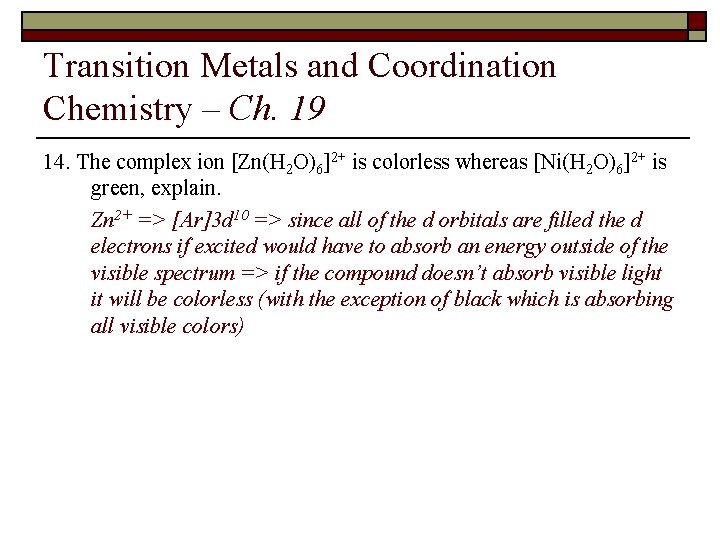
Transition Metals and Coordination Chemistry – Ch. 19 15. The complex ion [Zn(H 2 O)6]2+ is colorless whereas [Ni(H 2 O)6]2+ is green, explain.

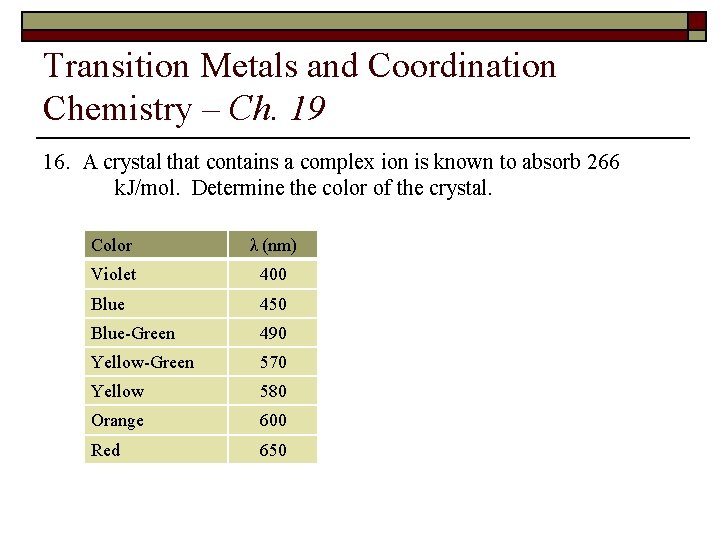
Transition Metals and Coordination Chemistry – Ch. 19 16. A crystal that contains a complex ion is known to absorb 266 k. J/mol. Determine the color of the crystal. Color λ (nm) Violet 400 Blue 450 Blue-Green 490 Yellow-Green 570 Yellow 580 Orange 600 Red 650

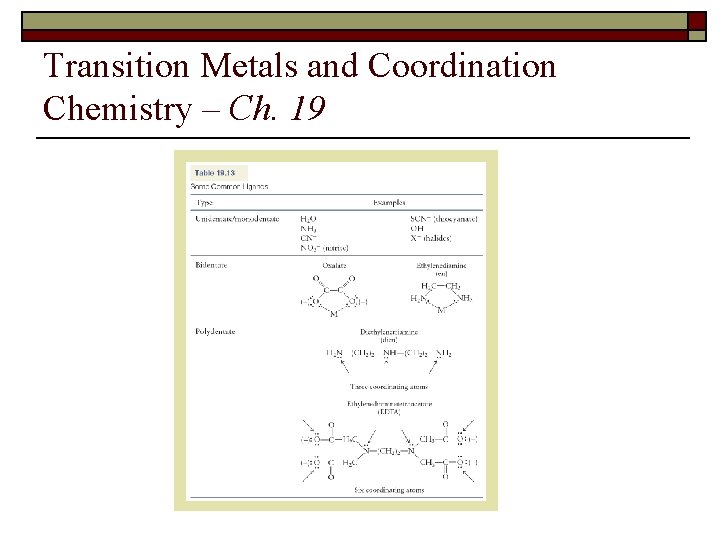
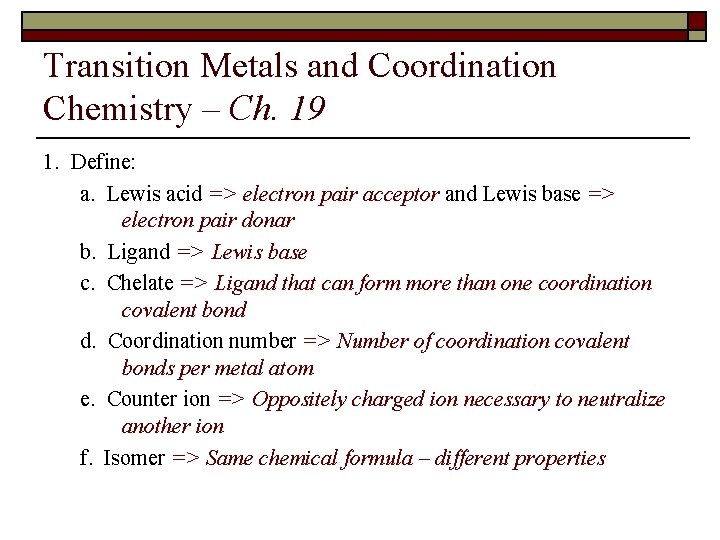
Transition Metals and Coordination Chemistry – Ch. 19 1. Define: a. Lewis acid => electron pair acceptor and Lewis base => electron pair donar b. Ligand => Lewis base c. Chelate => Ligand that can form more than one coordination covalent bond d. Coordination number => Number of coordination covalent bonds per metal atom e. Counter ion => Oppositely charged ion necessary to neutralize another ion f. Isomer => Same chemical formula – different properties

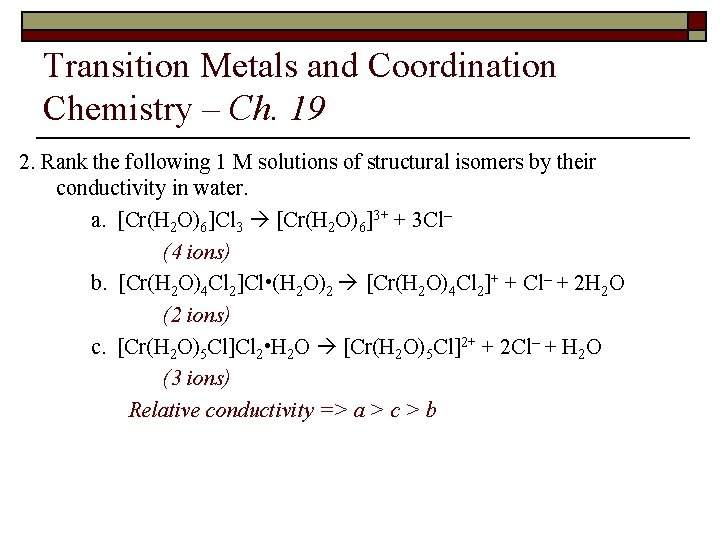
Transition Metals and Coordination Chemistry – Ch. 19 2. Rank the following 1 M solutions of structural isomers by their conductivity in water. a. [Cr(H 2 O)6]Cl 3 [Cr(H 2 O)6]3+ + 3 Cl– (4 ions) b. [Cr(H 2 O)4 Cl 2]Cl • (H 2 O)2 [Cr(H 2 O)4 Cl 2]+ + Cl– + 2 H 2 O (2 ions) c. [Cr(H 2 O)5 Cl]Cl 2 • H 2 O [Cr(H 2 O)5 Cl]2+ + 2 Cl– + H 2 O (3 ions) Relative conductivity => a > c > b

Transition Metals and Coordination Chemistry – Ch. 19 3. Name Formula lithium hexahydroxoferrate(III) Li 3[Fe(OH)6] diamminedichloroethylendiaminechromium(III) sulfate [Cr(NH 3)2 Cl 2(en)]2 SO 4 bis(ethylenediamine)oxalatomanganese(IV) nitrate [Mn(en)2(ox)](NO 3)2 or [Mn(NH 2 CH 2 NH 2)2(C 2 O 4)](NO 3)2 potassium dibromodicyanoplatinate(II) K 2[Pt. Br 2(CN)2]

Transition Metals and Coordination Chemistry – Ch. 19 4. Substance/Ion Ox # CN Name [Pt(NH 3)3 Br]+ +2 4 triamminebromoplatinum(II) ion K[Co. Br. Cl 3(en)] +3 6 potassium bromotrichloro(ethylenediamine)cobaltate(III) [Ni(CO)4] Br 2 +2 4 tetracarbonylnickel(II) bromide Na 2[Mn(C 2 O 4)2(CN)(NO 2)] +4 6 sodium cyanonitrobisoxalatomanganate(IV) [Cu(NH 3)4(H 2 O)2][Pt. I 6] +2, +4 6, 6 tetraamminediaquacopper(II) hexaiodoplatinate(IV)

Transition Metals and Coordination Chemistry – Ch. 19 5. _____ isomers and _____ isomers are classes of structural isomers. a. Geometric, optical b. Coordination, geometric c. Linkage, geometric d. Coordination, linkage e. Geometric, linkage 6. Which of the following complexes shows geometric isomerism? a. [Co(NH 3)5 Cl]SO 4 b. [Co(NH 3)6]Cl 3 c. Li[Co(NH 3)3 Cl 3] d. K[Co(NH 3)2 Cl 4] e. Na 3[Co. Cl 6]

Transition Metals and Coordination Chemistry – Ch. 19 7. Give the number of geometric isomers for the octahedral compound [MA 2 B 2 C 2], where A, B, and C represent ligands. a. 1 b. 2 c. 3 d. 5 e. 6 A A C A B C C B M M B C A B C C A A M M A C B B B A C

Transition Metals and Coordination Chemistry – Ch. 19 8. For the process Co(NH 3)5 Cl 2+ + Cl– ® Co(NH 3)4 Cl 2+ + NH 3, what would be the ratio of cis to trans isomers in the product? a. 1: 1 N N Cl b. 1: 2 N Cl Co Co Co c. 1: 4 Cl N N N d. 4: 1 N N Cl e. 2: 1 N N N Cl Cl Co N Cl N Co N Cl Cl Co N N Cl Cl N N N

Transition Metals and Coordination Chemistry – Ch. 19 9. Which of the following statements about the complex ion Co(en)2 Cl 2+ is true? (en = ethylenediamine, NH 2 CH 2 NH 2) a. The complex ion contains Co(I). b. The complex ion exhibits cis and trans geometric isomers, but no optical isomers. c. The complex ion exhibits two geometric isomers (cis and trans) and two optical isomers. d. Because en is a strong field ligand (large Δ), the complex ion is paramagnetic. e. The geometric isomers of the complex ion have identical chemical properties.

Transition Metals and Coordination Chemistry – Ch. 19 10. A solution is made by dissolving 0. 875 g of Co(NH 3)4 Cl 3 in 25. 0 g of water. The solution freezes at -0. 56 °C. (Kf = 1. 86 °C kg/mol) Calculate the number of ions (i) produced when Co(NH 3)4 Cl 3 is dissolved in water. ΔTf = - kfim -0. 56 C = -(1. 86 Ckg/mol)(i)(0. 875 g/233. 44 g/mol)/(0. 025 kg) i = 2 => [Co(NH 3)4 Cl 2]Cl

Transition Metals and Coordination Chemistry – Ch. 19 11. An orange complex and a violet complex were both found to have ferric ion as their central metal; which one absorbs light with a longer wavelength? The orange complex absorbs blue light and the violet complex absorbs yellow light – λyellow > λblue – so the violet complex absorbs the longer wavelength

Transition Metals and Coordination Chemistry – Ch. 19 12. Draw the d orbital diagrams according to the crystal field model in order to predict how many unpaired electrons are in each of the following: a. [ Fe(CN)6]4 b. [Co. Br 6]3 c. [Fe(H 2 O)4]3+ (tetrahedral)
![Transition Metals and Coordination Chemistry Ch 19 13 Solutions of CoNH 363 Transition Metals and Coordination Chemistry – Ch. 19 13. Solutions of [Co(NH 3)6]3+ ,](https://slidetodoc.com/presentation_image_h/1cc8a71a78acbc2aa57f007255d7aeb2/image-32.jpg)
Transition Metals and Coordination Chemistry – Ch. 19 13. Solutions of [Co(NH 3)6]3+ , [Co(NO 2)6]3 -and [Co. F 6]3 - are colored. The solutions were red, yellow and blue. Match the solution to the color. Color Seen Color Absorbed Relative Wavelength Relative Energy Relative Ligand Strength Ligand red green medium NH 3 yellow violet shortest highest strongest NO 2 - blue orange longest lowest weakest F-

Transition Metals and Coordination Chemistry – Ch. 19 14. The complex ion [Zn(H 2 O)6]2+ is colorless whereas [Ni(H 2 O)6]2+ is green, explain. Zn 2+ => [Ar]3 d 10 => since all of the d orbitals are filled the d electrons if excited would have to absorb an energy outside of the visible spectrum => if the compound doesn’t absorb visible light it will be colorless (with the exception of black which is absorbing all visible colors)