Transition Metals and Coordination Chemistry Chapter 23 Transition










![Coordination Compound Consist of a complex ion and necessary counter ions [Co(NH 3)5 Cl]Cl Coordination Compound Consist of a complex ion and necessary counter ions [Co(NH 3)5 Cl]Cl](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-21.jpg)
![[Co(NH 3)6]Cl 3 [Pt(NH 3)4]Br 2 Complex ion remains intact upon dissolution in water [Co(NH 3)6]Cl 3 [Pt(NH 3)4]Br 2 Complex ion remains intact upon dissolution in water](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-22.jpg)









![Examples K 2[Co(NH 3)2 Cl 4] potassium diamminetetrachlorocobaltate(II) [Co(NH 3)4 Cl 2]Cl tetraamminedichlorocobalt(III) chloride Examples K 2[Co(NH 3)2 Cl 4] potassium diamminetetrachlorocobaltate(II) [Co(NH 3)4 Cl 2]Cl tetraamminedichlorocobalt(III) chloride](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-32.jpg)



![Linkage Isomers [Co(NH 3)5(NO 2)]Cl 2 Pentaamminenitrocobalt(III) chloride [Co(NH 3)5(ONO)]Cl 2 Pentaamminenitritocobalt(III) chloride Linkage Isomers [Co(NH 3)5(NO 2)]Cl 2 Pentaamminenitrocobalt(III) chloride [Co(NH 3)5(ONO)]Cl 2 Pentaamminenitritocobalt(III) chloride](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-38.jpg)















![[V(H 2 O)6]2+ [V(H 2 O)6]3+ [Cr(NH 3)5 Cl]2+s [V(H 2 O)6]2+ [V(H 2 O)6]3+ [Cr(NH 3)5 Cl]2+s](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-58.jpg)






- Slides: 67

Transition Metals and Coordination Chemistry Chapter 23

Transition Metals Similarities within a given period and within a given group. Last electrons added are inner electrons (d’s, f’s).









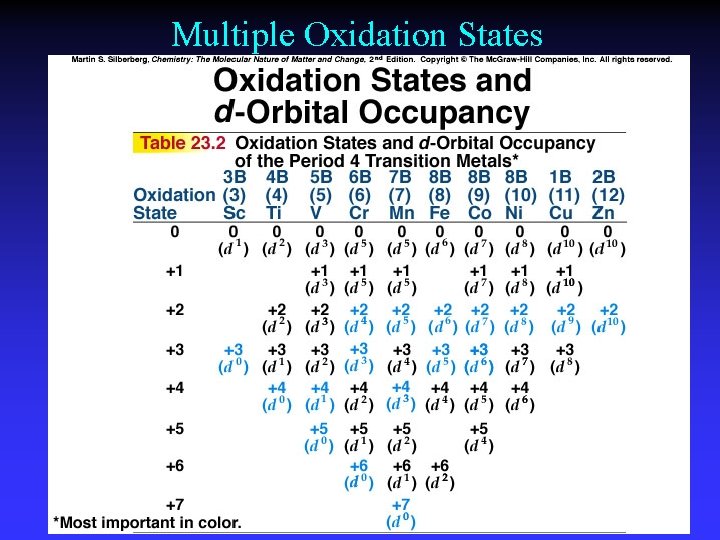
Multiple Oxidation States

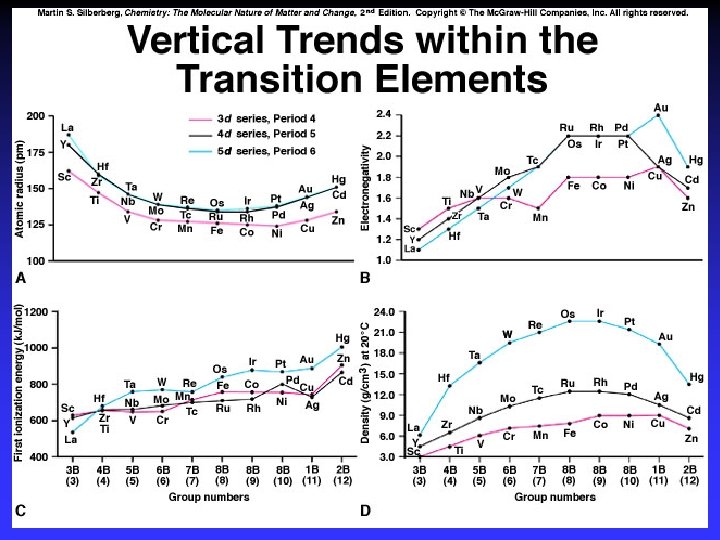
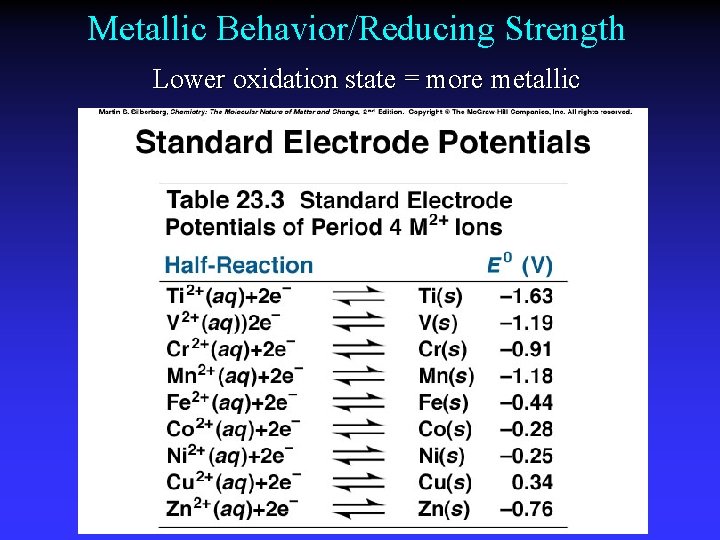
Metallic Behavior/Reducing Strength Lower oxidation state = more metallic

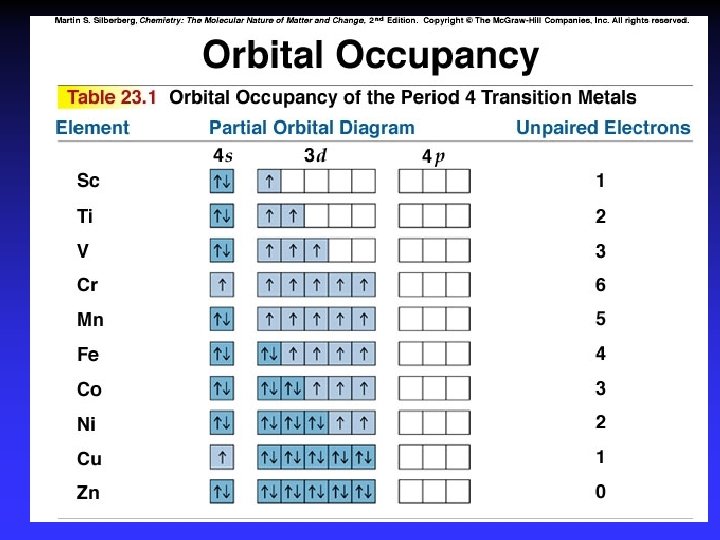
Color and Magnetism e- in partially filled d sublevel absorbs visible light moves to slightly higher energy d orbital Magnetic properties due to unpaired electrons

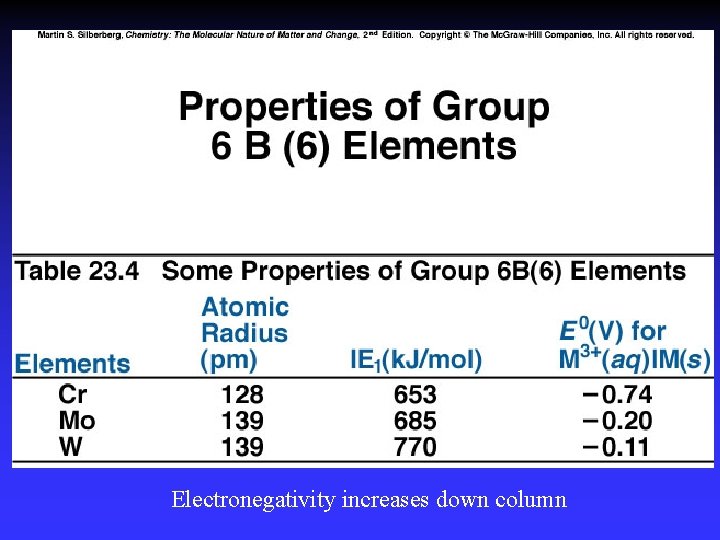
Electronegativity increases down column

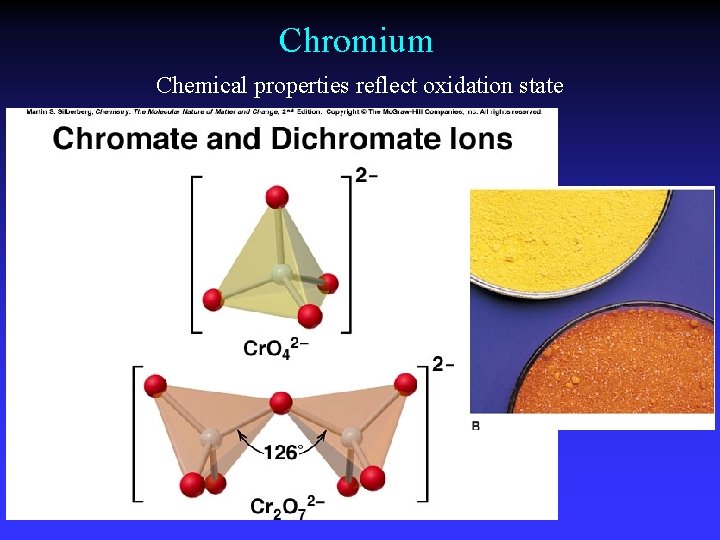
Chromium Chemical properties reflect oxidation state

Valence-State Electronegativity, EN: electron “pulling power” Valence-state EN: metal in higher oxidation state is more positive has stronger pull on electrons is more electronegative “Effective EN”

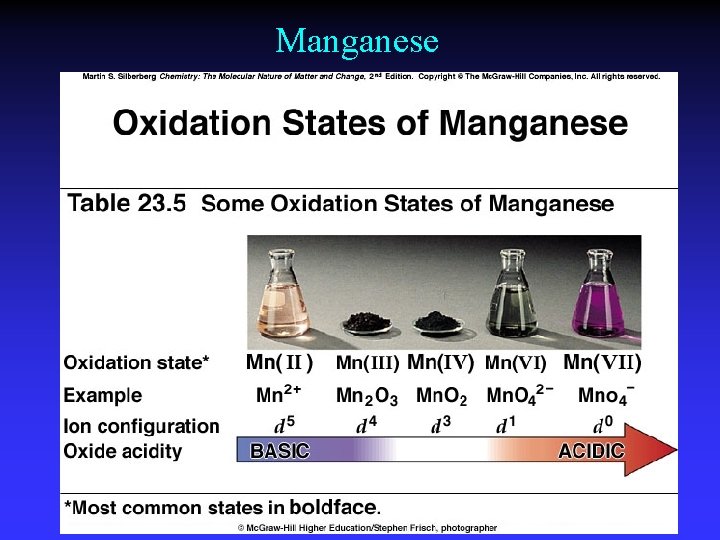
Manganese

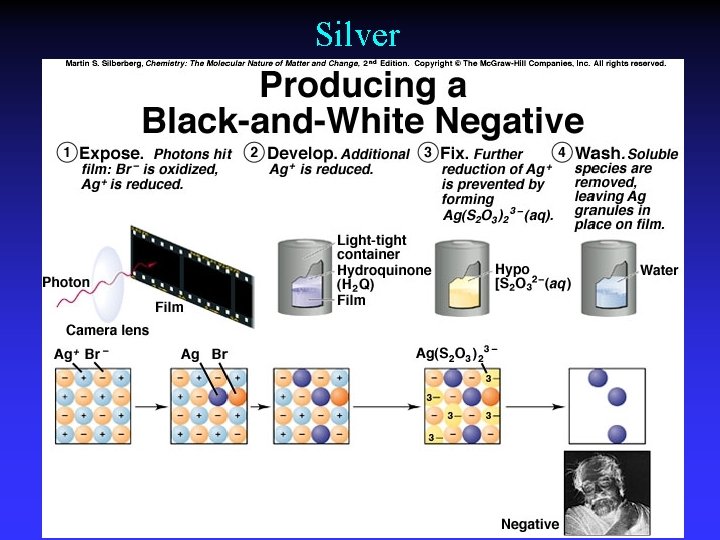
Silver

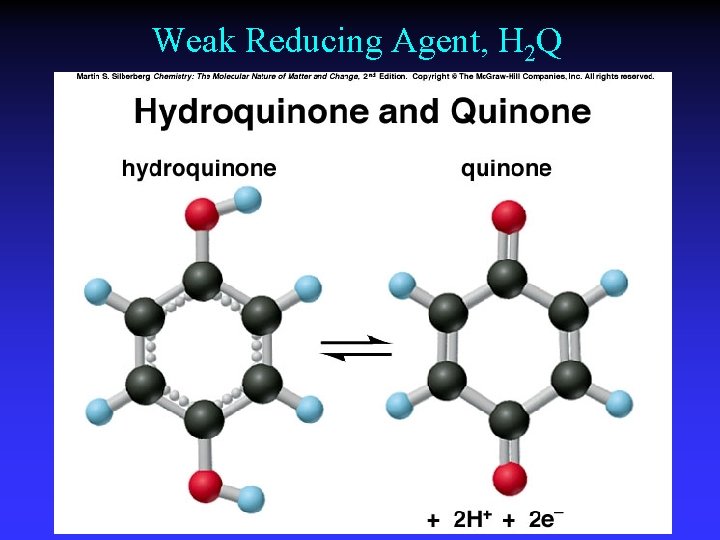
Weak Reducing Agent, H 2 Q

Mercury
![Coordination Compound Consist of a complex ion and necessary counter ions CoNH 35 ClCl Coordination Compound Consist of a complex ion and necessary counter ions [Co(NH 3)5 Cl]Cl](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-21.jpg)
Coordination Compound Consist of a complex ion and necessary counter ions [Co(NH 3)5 Cl]Cl 2 Complex ion: [Co(NH 3)5 Cl]2+ Co 3+ + 5 NH 3 + Cl= 1(3+) + 5 (0) + 1(1 -) = 2+ Counter ions: 2 Cl-
![CoNH 36Cl 3 PtNH 34Br 2 Complex ion remains intact upon dissolution in water [Co(NH 3)6]Cl 3 [Pt(NH 3)4]Br 2 Complex ion remains intact upon dissolution in water](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-22.jpg)
[Co(NH 3)6]Cl 3 [Pt(NH 3)4]Br 2 Complex ion remains intact upon dissolution in water

Complex Ion Species where transition metal ion is surrounded by a certain number of ligands. Transition metal ion: Ligands: Lewis acid Lewis bases Co(NH 3)63+ Pt(NH 3)3 Br+


Ligands Molecule or ion having a lone electron pair that can be used to form a bond to a metal ion (Lewis base). coordinate covalent bond: metal-ligand bond monodentate: bidentate: polydentate: one bond to metal ion two bond to metal ion more than two bonds to a metal ion possible



Formulas of Coordination Compounds 1. Cation then anion 2. Total charges must balance to zero 3. Complex ion in brackets K 2[Co(NH 3)2 Cl 4] [Co(NH 3)4 Cl 2]Cl

Names of Coordination Compounds 1. Cation then anion 2. Ligands in alphabetical order before metal ion neutral: molecule name* anionic: -ide -o prefix indicates number of each 3. Oxidation state of metal ion in () only if more than one possible 4. If complex ion = anion, metal ending -ate


![Examples K 2CoNH 32 Cl 4 potassium diamminetetrachlorocobaltateII CoNH 34 Cl 2Cl tetraamminedichlorocobaltIII chloride Examples K 2[Co(NH 3)2 Cl 4] potassium diamminetetrachlorocobaltate(II) [Co(NH 3)4 Cl 2]Cl tetraamminedichlorocobalt(III) chloride](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-32.jpg)
Examples K 2[Co(NH 3)2 Cl 4] potassium diamminetetrachlorocobaltate(II) [Co(NH 3)4 Cl 2]Cl tetraamminedichlorocobalt(III) chloride




Structural Isomerism 1 Coordination isomerism: Composition of the complex ion varies. [Cr(NH 3)5 SO 4]Br and [Cr(NH 3)5 Br]SO 4

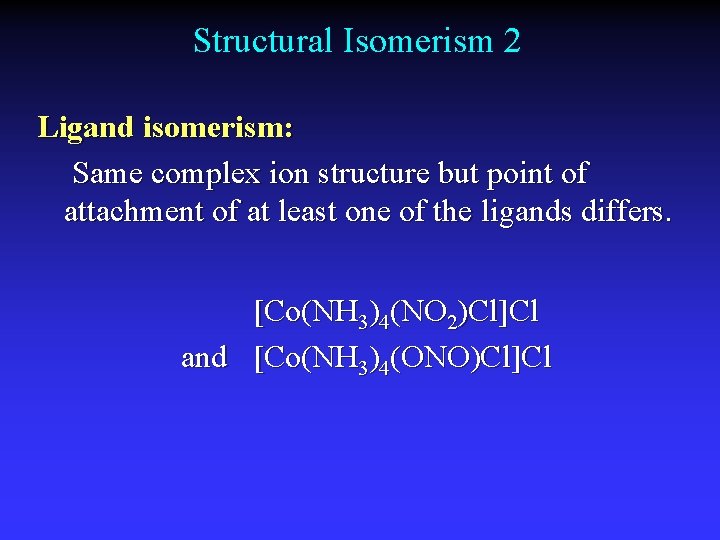
Structural Isomerism 2 Ligand isomerism: Same complex ion structure but point of attachment of at least one of the ligands differs. [Co(NH 3)4(NO 2)Cl]Cl and [Co(NH 3)4(ONO)Cl]Cl
![Linkage Isomers CoNH 35NO 2Cl 2 PentaamminenitrocobaltIII chloride CoNH 35ONOCl 2 PentaamminenitritocobaltIII chloride Linkage Isomers [Co(NH 3)5(NO 2)]Cl 2 Pentaamminenitrocobalt(III) chloride [Co(NH 3)5(ONO)]Cl 2 Pentaamminenitritocobalt(III) chloride](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-38.jpg)
Linkage Isomers [Co(NH 3)5(NO 2)]Cl 2 Pentaamminenitrocobalt(III) chloride [Co(NH 3)5(ONO)]Cl 2 Pentaamminenitritocobalt(III) chloride

Stereoisomerism 1 Geometric isomerism (cis-trans): Atoms or groups arranged differently spatially relative to metal ion Pt(NH 3)2 Cl 2



Stereoisomerism 2 Optical isomerism: Have opposite effects on plane-polarized light (no superimposable mirror images)








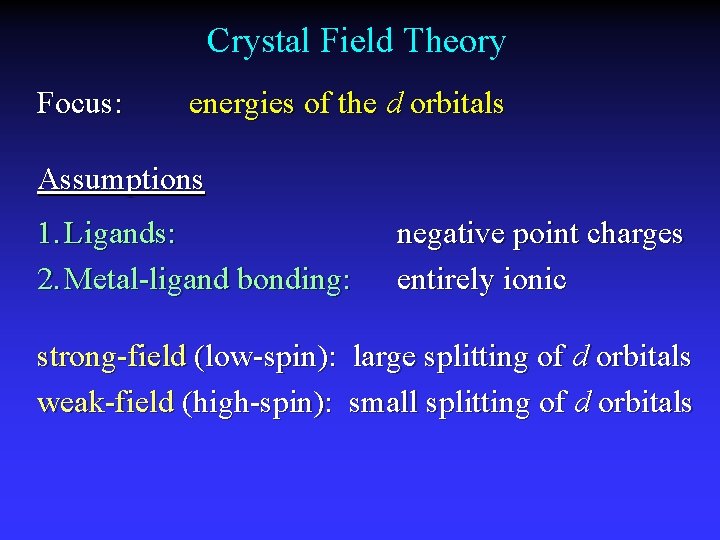
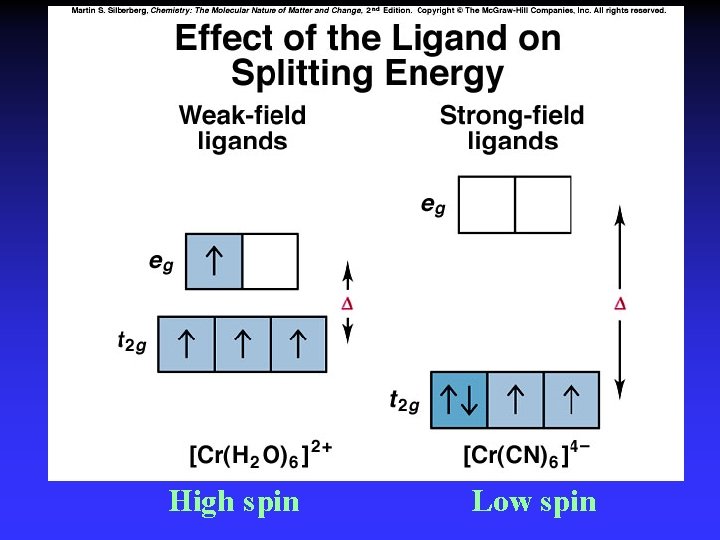
Crystal Field Theory Focus: energies of the d orbitals Assumptions 1. Ligands: 2. Metal-ligand bonding: negative point charges entirely ionic strong-field (low-spin): large splitting of d orbitals weak-field (high-spin): small splitting of d orbitals



D = crystal field splitting

High spin Low spin



![VH 2 O62 VH 2 O63 CrNH 35 Cl2s [V(H 2 O)6]2+ [V(H 2 O)6]3+ [Cr(NH 3)5 Cl]2+s](https://slidetodoc.com/presentation_image/4a1ee8012f8481e97357305a4b6c4698/image-58.jpg)
[V(H 2 O)6]2+ [V(H 2 O)6]3+ [Cr(NH 3)5 Cl]2+s




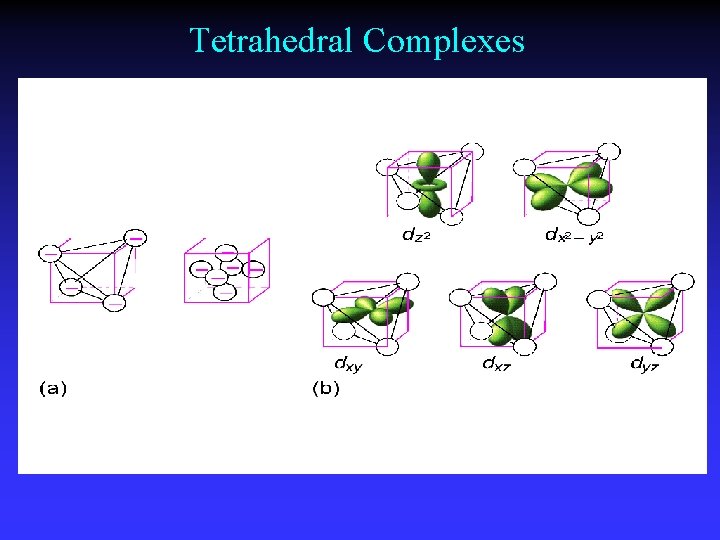
Tetrahedral Complexes


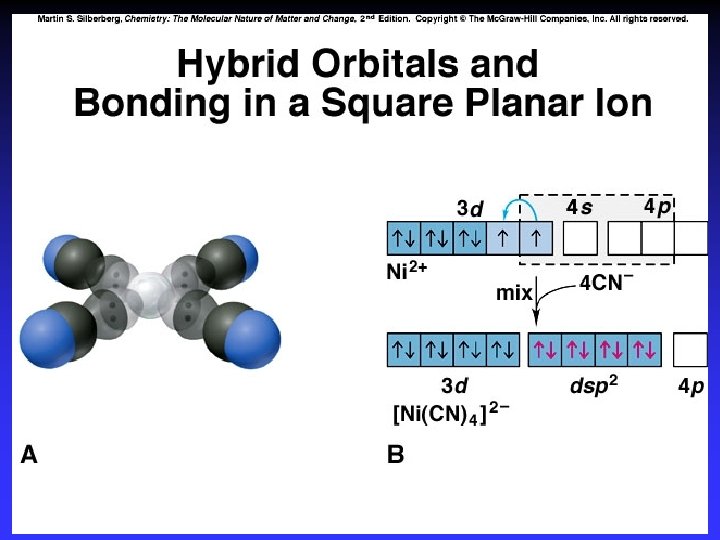
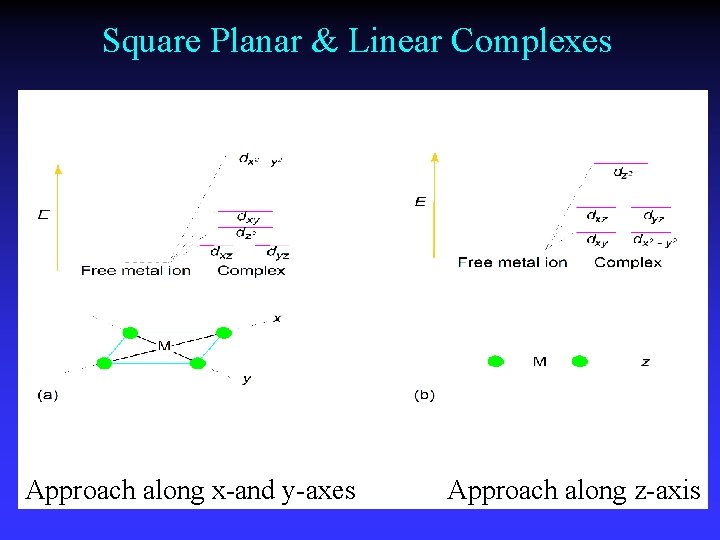
Square Planar & Linear Complexes Approach along x-and y-axes Approach along z-axis



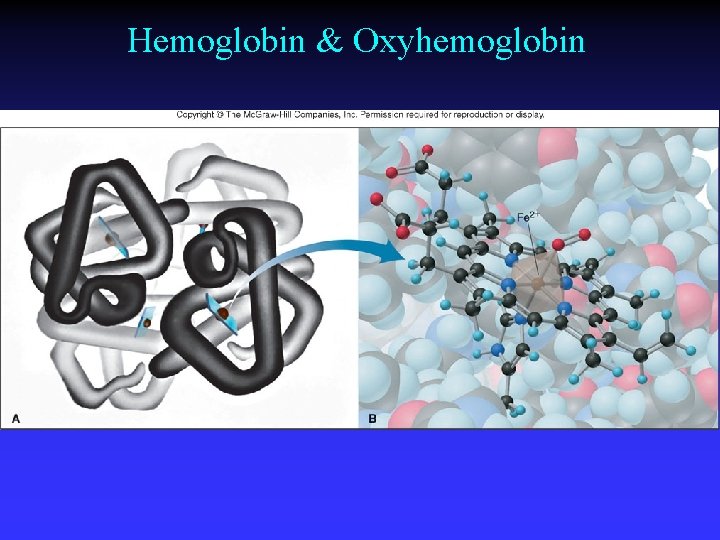
Hemoglobin & Oxyhemoglobin