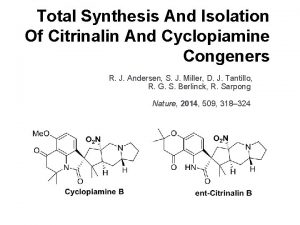
Total Synthesis of Rapamycin Isolation and Structure Determination































- Slides: 45

Total Synthesis of Rapamycin Isolation and Structure Determination: Vézina, C. ; Kudelski, A. ; Sehgal, S. N. J. Antibiotics 1975, 28, 721. Swindells, D. C. N. ; White, P. S. ; Findlay, J. A. Can. J. Chem. 1978, 56, 2491. Findlay, J. A. ; Radics, L. Can. J. Chem. 1981, 59, 49. Mc. Alpine, J. B. ; Swanson, S. J. ; Jackson, M. ; Whittern, D. N. J. Antibiotics 1991, 44, C-3. Total Syntheses: Nicolaou, K. C. ; Chakraborty, T. K. ; Piscopio, A. D. ; Minowa, N. ; Bertinato, P. J. Am. Chem. Soc. 1993, 115, 4419. Hayward, C. M. ; Yohannes, D. ; Danishefsky, S. J. J. Am. Chem. Soc. 1993, 115, 9345. Romo, D. ; Meyer, S. D. ; Johnson, D. D. ; Schreiber, S. L. J. Am. Chem. Soc. 1993, 115, 7906. Smith, A. B. , III; Condon, S. M. ; Mc. Cauley, J. A. ; Leazer, J. L. , Jr. ; Leahy, J. W. ; Maleczka, R. E. , Jr. J. Am. Chem. Soc. 1995, 117, 5407 -5408.

Immunomodulators rapamycin FK-506 cyclosporin A

Rapamycin’s Mechanism of Action IL-2 Receptor The Cell Cycle Restriction Point ? G 1 p 70 S 6 Kinase S G 0 M Cdc 2 Kinase G 2 40 S Ribosomal Protein S 6 Schreiber, S. L. ; Albers, M. W. ; Brown, E. J. Acc. Chem. Res. 1993, 26, 412. Chung, J. ; Kuo, C. J. ; Crabtree, G. R. ; Blenis, J. Cell 1992, 69, 1227.

KCN's Retrosynthetic Analysis of Rapamycin rapamycin

Synthesis of Oxazolidone A

Synthesis of Oxazolidone A (continued)

KCN's Retrosynthetic Analysis of Rapamycin rapamycin

Synthesis of Subunit B Z-enolate

Synthesis of Subunit B (continued)

KCN's Retrosynthetic Analysis of Rapamycin rapamycin

Synthesis of Vinyliodide D

Synthesis of Vinyliodide D (continued)

KCN's Retrosynthetic Analysis of Rapamycin rapamycin

The Union of A + B + E

Elaboration of EAB

The Introduction of D rapamycin EABD

The End Game – Tricarbonyl Formation Note: the first HF step removes the TES groups and the second HF step removes the TIPS groups

The End Game – The “Stitching” Stille Reaction rapamycin

Summary • Completed the first total synthesis of (-)-rapamycin. – The longest linear sequence from an article of commerce consists of thirty-seven steps. – The longest linear sequence from our five sub-targets is sixteen steps. – Total steps: 102 • Instructional applications of the Stille reaction, oxidation chemistry, chiral auxiliaries, organosilicons, protective groups, etc.

Smith’s Retrosynthetic Analysis of Rapamycin and Demethoxyrapamycin

Synthesis of Iodide A

Synthesis of Dithiane B

Synthesis of Dithiane C

Retrosynthetic Analysis of Rapamycin

Synthesis of the Ortho Ester Exploitation of Alternate Ortho Ester Diastereomer Employed in Smith’s Latrunculin Synthetic Venture

Synthesis of the E and Z Eneynes

Mechanism of Olefin Isomerization

Stereochemistry of Eneyne Addition to Aldehyde

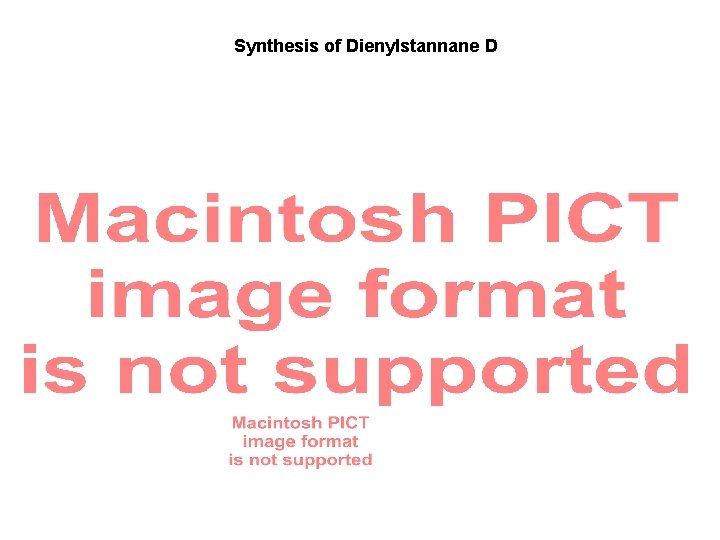
Synthesis of Dienylstannane D

Retrosynthetic Analysis of Rapamycin

Construction of a C 27 -C 42 Aldehyde

Construction of the C 22 -C 42 Subunit

Synthesis of Demethoxyrapamycin: Construction of Advanced ABC Intermediate

Retrosynthetic Analysis of Rapamycin and Demethoxyrapamycin: Introduction of the Tricarbonyl Segment

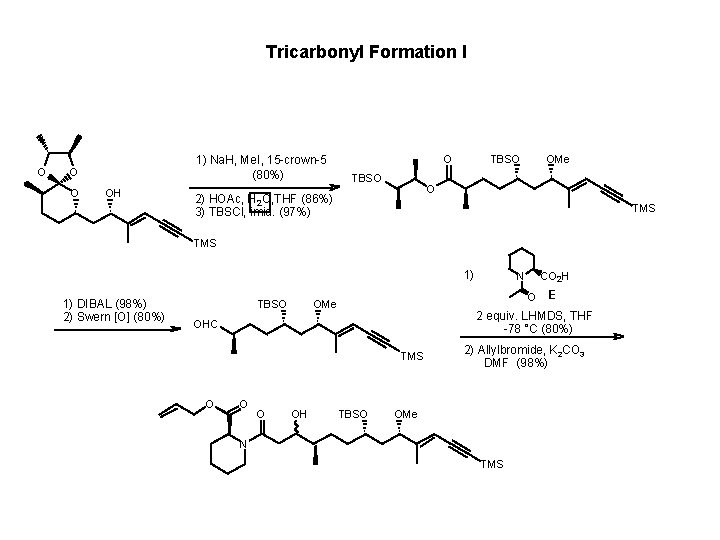
Tricarbonyl Formation I O 1) Na. H, Me. I, 15 -crown-5 (80%) O O OH O TBSO OMe O 2) HOAc, H 2 O, THF (86%) 3) TBSCl, imid. (97%) TMS 1) 1) DIBAL (98%) 2) Swern [O] (80%) TBSO O OH E 2 equiv. LHMDS, THF -78 °C (80%) TMS O CO 2 H O OMe OHC O N TBSO 2) Allylbromide, K 2 CO 3 DMF (98%) OMe N TMS

Tricarbonyl Formation II

Pipecolinyl Acylation

Proposed Endgame: Bis-Hydrostannylation

Attempted Macrocyclizations

Preparation of ABC vinylstannane & DE vinyl iodide

Proposed Endgame Strategy for the Total Synthesis of Rapamycin and Demethoxyrapamycin

Macrocyclization

Demethoxyrapamycin

Rapamycin

Summary • Developed a highly convergent and efficient total synthesis of (-)-rapamycin. – The longest linear sequence from an article of commerce consists of thirty-three steps. – The longest linear sequence from our five sub-targets is fourteen steps. – After the coupling of the C(1)-C(20) fragment to the C(22)C(42) fragment only three steps are required to complete the synthesis. • Completed the first total synthesis of demethoxyrapamycin. – The synthesis serves as a structure proof. – The synthesis establishes our unified synthetic approach as being amenable to the preparation of analogs.
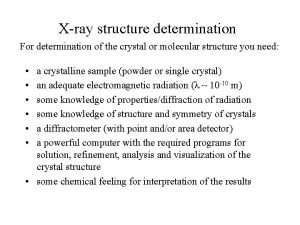
 X-ray structure determination
X-ray structure determination Anomalous
Anomalous Uafir fórmula
Uafir fórmula Total revenues minus total costs equals
Total revenues minus total costs equals Total revenues minus total costs equals
Total revenues minus total costs equals Total revenues minus total costs equals
Total revenues minus total costs equals Total revenue minus total expenses
Total revenue minus total expenses Paragraph synthesis
Paragraph synthesis Curleys wife loneliness
Curleys wife loneliness Punnett square for sex linked traits
Punnett square for sex linked traits Heterogametic
Heterogametic Determination and description of material quality
Determination and description of material quality Price and output determination under oligopoly
Price and output determination under oligopoly Sex determination and sex linkage
Sex determination and sex linkage Spi in software engineering
Spi in software engineering Price and output determination under monopoly
Price and output determination under monopoly Exchange rate determination and forecasting
Exchange rate determination and forecasting Price discovery and price determination
Price discovery and price determination Example of invisibility bias
Example of invisibility bias Example of emphasis by isolation
Example of emphasis by isolation Secure architecture principles isolation and less
Secure architecture principles isolation and less Aries in dbms
Aries in dbms Chapter 19 an age of explorations and isolation
Chapter 19 an age of explorations and isolation Isolation of pure cultures
Isolation of pure cultures Dutch exploration routes
Dutch exploration routes Mercantilism
Mercantilism Principle of least common mechanism
Principle of least common mechanism Linear regression riddle a answer key
Linear regression riddle a answer key Blank determination example
Blank determination example Brainpop sex
Brainpop sex Self-determination worksheets pdf
Self-determination worksheets pdf Computing sample size
Computing sample size Shipping point determination
Shipping point determination Shipping point determination sap
Shipping point determination sap R squared interpretation
R squared interpretation Requirement determination example
Requirement determination example Correlation stufy
Correlation stufy Metodo di lowry
Metodo di lowry Determination in sanskrit
Determination in sanskrit Author tone
Author tone Grit personality trait
Grit personality trait How to read microhematocrit reader
How to read microhematocrit reader Coefficient of determination interpretation
Coefficient of determination interpretation Standard error of regression
Standard error of regression How to find lsrl
How to find lsrl Gravimetric determination of phosphorus in plant food
Gravimetric determination of phosphorus in plant food