Determination of Total Nitrogen By Kjeldahls Method Determination

Determination of Total Nitrogen By Kjeldahl’s Method
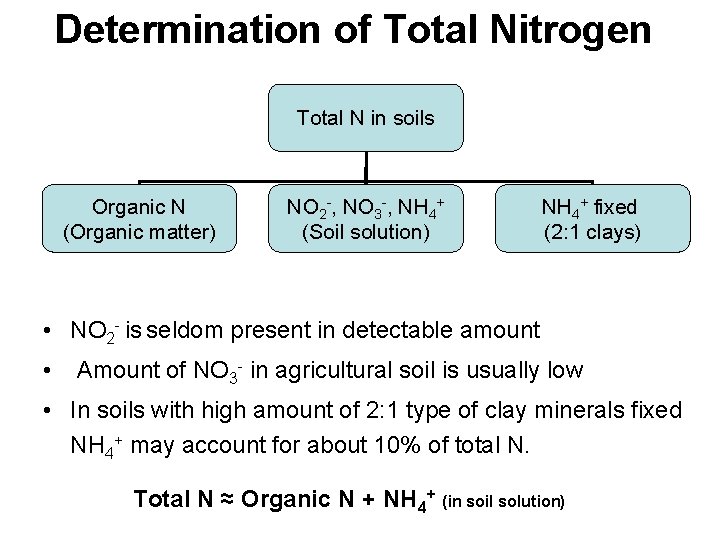
Determination of Total Nitrogen Total N in soils Organic N (Organic matter) NO 2 -, NO 3 -, NH 4+ (Soil solution) NH 4+ fixed (2: 1 clays) • NO 2 - is seldom present in detectable amount • Amount of NO 3 - in agricultural soil is usually low • In soils with high amount of 2: 1 type of clay minerals fixed NH 4+ may account for about 10% of total N. Total N ≈ Organic N + NH 4+ (in soil solution)

Methods of total N determination • 1) Dumas method (1831) - sample is combusted with Cu. O at high temperature to liberate gases (N 2 O and CO 2). - gas evolved is passes through strong alkaline to absorb CO 2. - N 2 O gas is passes over hot Cu to form N 2 - N 2 gas produced is measured to estimate total N - Dry oxidation process

Methods of total N determination 2) Kjeldahl’s Method (1833) - Samples are digested with H 2 SO 4 to convert N in the sample to (NH 4)2 SO 4. - Determination of the NH 4+ in the digest by distillation and titration. - Wet oxidation process.
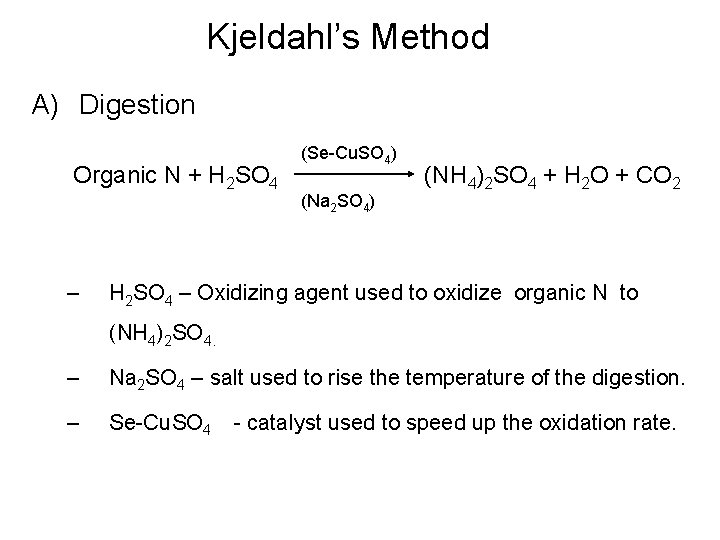
Kjeldahl’s Method A) Digestion Organic N + H 2 SO 4 – (Se-Cu. SO 4) (Na 2 SO 4) (NH 4)2 SO 4 + H 2 O + CO 2 H 2 SO 4 – Oxidizing agent used to oxidize organic N to (NH 4)2 SO 4. – Na 2 SO 4 – salt used to rise the temperature of the digestion. – Se-Cu. SO 4 - catalyst used to speed up the oxidation rate.

Kjeldahl’s Method Digestion procedure 1. 2. 3. 4. 5. 6. 7. 8. 9. Weigh 10 g soil into 500 ml Kjeldahl flask. Add 10 ml distilled water and leave to stand for 10 mins. Add the catalyst Add 2 tablets Na 2 SO 4 Add 20 ml Conc. H 2 SO 4 Digest until clear and colourless (1 – 1½ hrs). Further digest for 30 -45 mins. Allow the flask to cool. Decant or filter the fluid into a 100 ml volumetric flask washing the sand with altogether 50 ml distilled water, small at a time. 10. Make up to the mark with distilled water.
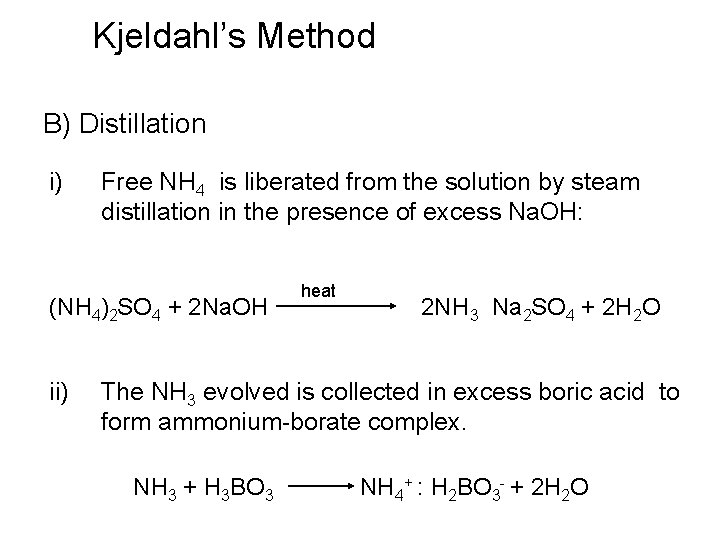
Kjeldahl’s Method B) Distillation i) Free NH 4 is liberated from the solution by steam distillation in the presence of excess Na. OH: (NH 4)2 SO 4 + 2 Na. OH ii) heat 2 NH 3 Na 2 SO 4 + 2 H 2 O The NH 3 evolved is collected in excess boric acid to form ammonium-borate complex. NH 3 + H 3 BO 3 NH 4+ : H 2 BO 3 - + 2 H 2 O

Kjeldahl’s Method B) Distillation procedure 1. Transfer an aliquot of 10 ml of the liquid into the Kjeldahl apparatus 2. Add 15 ml of the strong Na. OH. 3. Distil over steam for 10 minutes into 10 ml of the Boric acid in a 500 ml conical flask.

Kjeldahl’s Method C) Titration The amount of NH 3 liberated and captured by boric acid is determined by titrating with 0. 1 N HCl NH 4+ : H 2 BO 3 - + HCl NH 4 Cl + H 3 BO 3 Procedure 1. 2. 3. Add 8 drops of the mixed indicator. Titrate with 0. IN HCl till colour changes to grey and then suddenly flashes to pink. Carry out blank determination with distilled water.
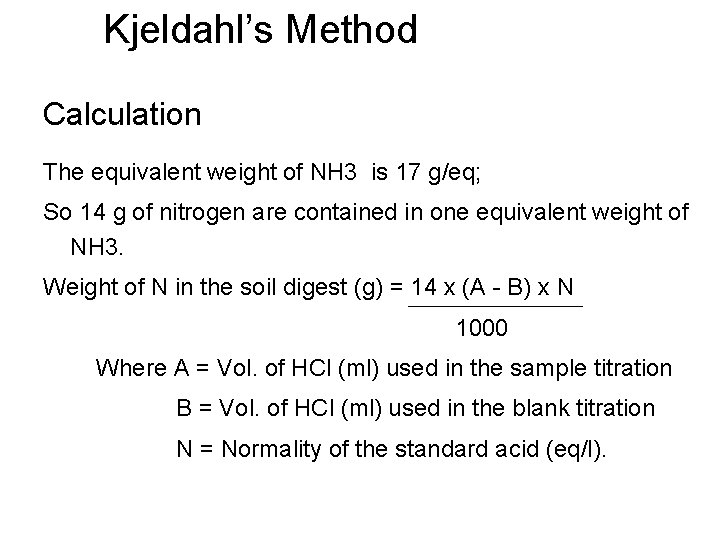
Kjeldahl’s Method Calculation The equivalent weight of NH 3 is 17 g/eq; So 14 g of nitrogen are contained in one equivalent weight of NH 3. Weight of N in the soil digest (g) = 14 x (A - B) x N 1000 Where A = Vol. of HCl (ml) used in the sample titration B = Vol. of HCl (ml) used in the blank titration N = Normality of the standard acid (eq/l).
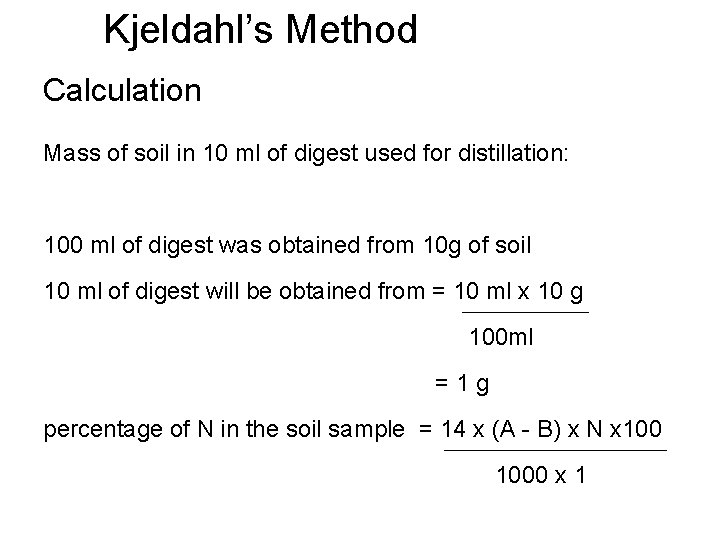
Kjeldahl’s Method Calculation Mass of soil in 10 ml of digest used for distillation: 100 ml of digest was obtained from 10 g of soil 10 ml of digest will be obtained from = 10 ml x 10 g 100 ml =1 g percentage of N in the soil sample = 14 x (A - B) x N x 1000 x 1

Kjeldahl’s Method Calculation i) ppm N = % N x 104 ii) Ib N/acre F. S = ppm N x 2 iii) kg N/ha = ib N/acre F. S x 1. 12
- Slides: 12