Thermodynamics Lecture Series Reference Chap 20 Halliday Resnick







- Slides: 34

Thermodynamics Lecture Series Reference: Chap 20 Halliday & Resnick Fundamental of Physics 6 th edition Kinetic Theory of Gases – Microscopic Thermodynamics Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail. com http: //www 3. uitm. edu. my/staff/drjj/ Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 1

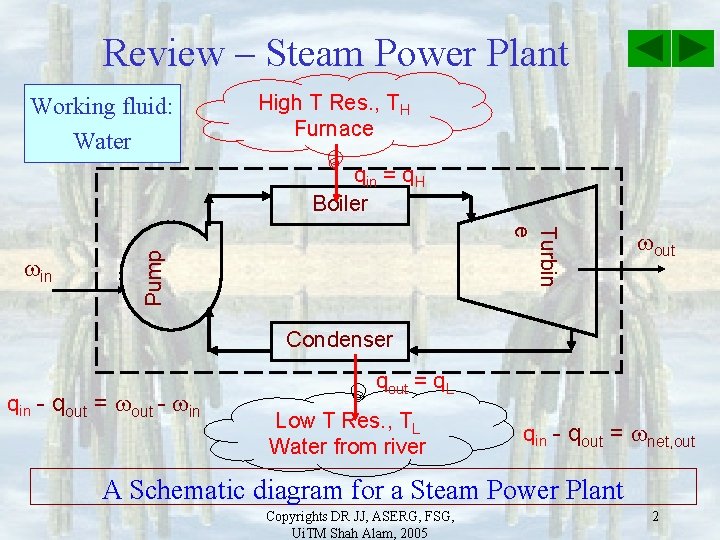
Review – Steam Power Plant Working fluid: Water High T Res. , TH Furnace qin = q. H Boiler Pump Turbin e in out Condenser qin - qout = out - in qout = q. L Low T Res. , TL Water from river qin - qout = net, out A Schematic diagram for a Steam Power Plant Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 2

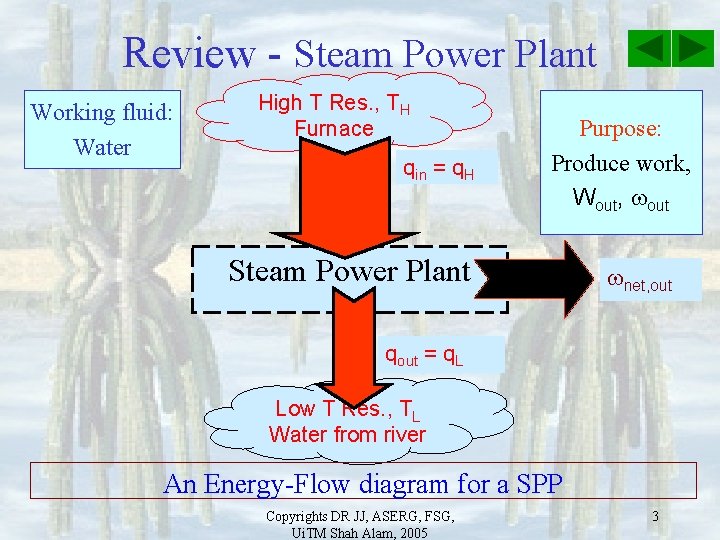
Review - Steam Power Plant Working fluid: Water High T Res. , TH Furnace qin = q. H Purpose: Produce work, Wout, out Steam Power Plant net, out qout = q. L Low T Res. , TL Water from river An Energy-Flow diagram for a SPP Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 3

Review - Steam Power Plant Thermal Efficiency for steam power plants For real engines, need to find q. L and q. H. Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 4

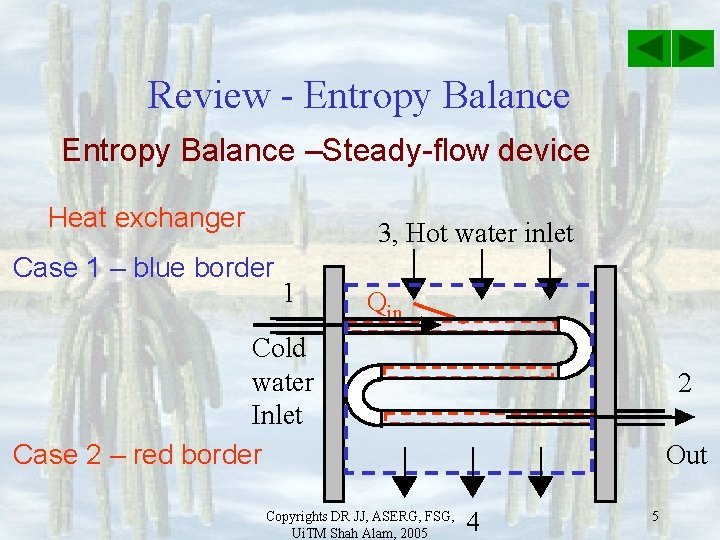
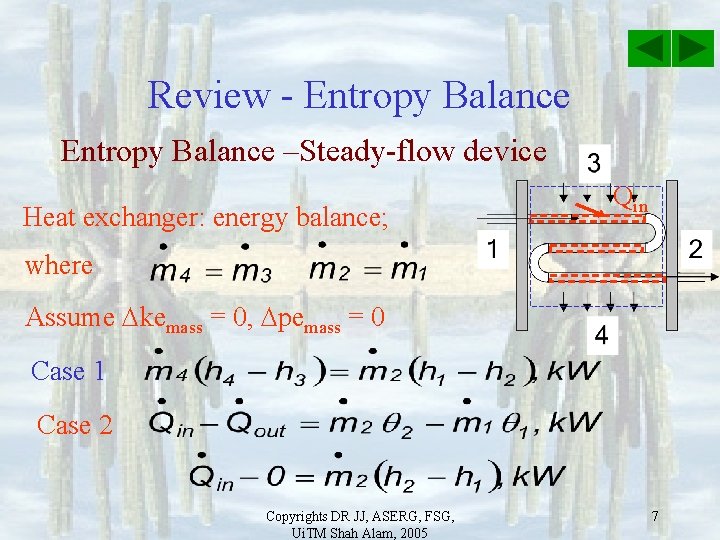
Review - Entropy Balance –Steady-flow device Heat exchanger 3, Hot water inlet Case 1 – blue border 1 Qin Cold water Inlet Case 2 – red border Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 2 Out 4 5

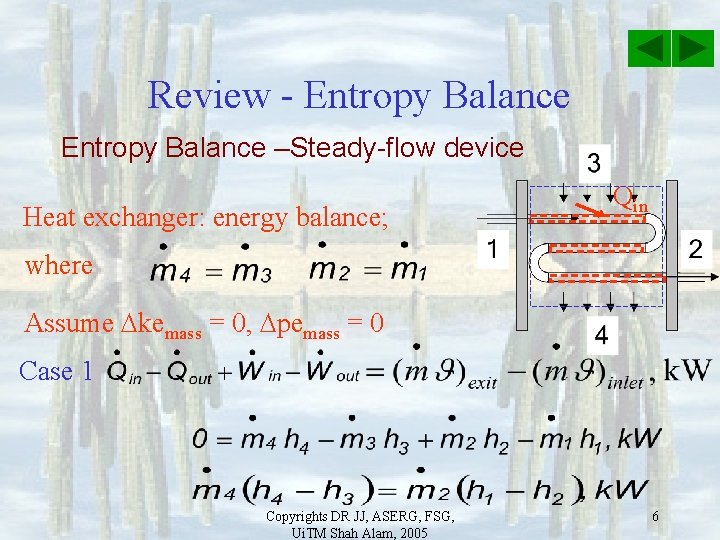
Review - Entropy Balance –Steady-flow device Heat exchanger: energy balance; Qin where Assume kemass = 0, pemass = 0 Case 1 Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 6

Review - Entropy Balance –Steady-flow device Heat exchanger: energy balance; Qin where Assume kemass = 0, pemass = 0 Case 1 Case 2 Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 7

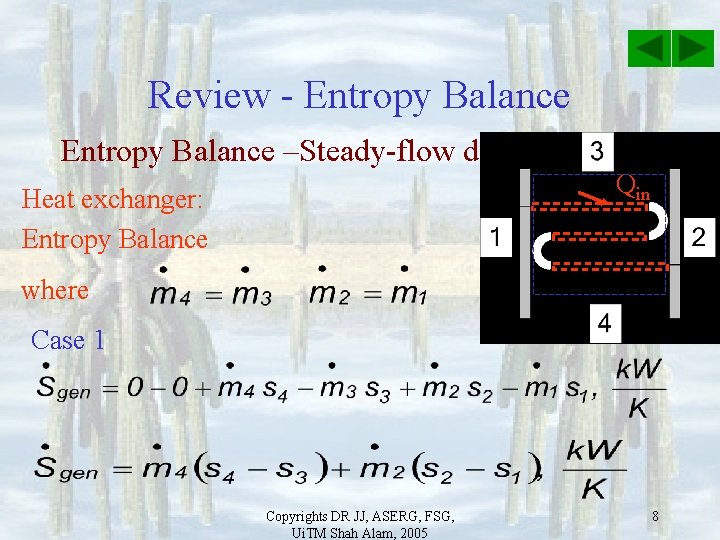
Review - Entropy Balance –Steady-flow device Qin Heat exchanger: Entropy Balance where Case 1 Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 8

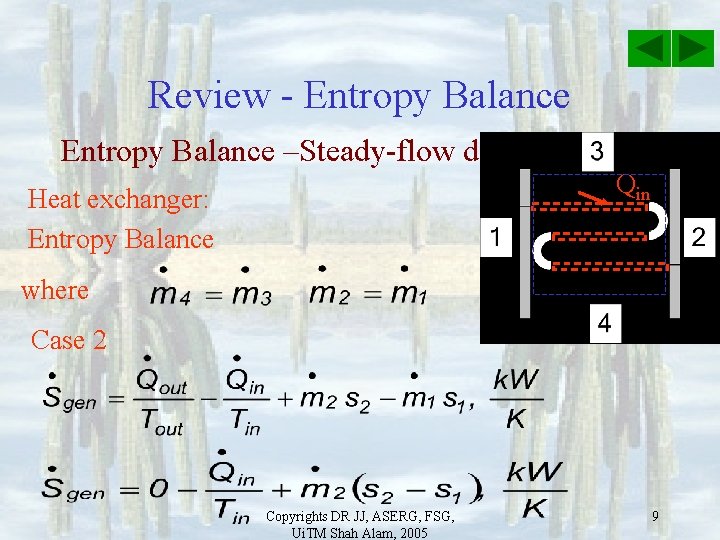
Review - Entropy Balance –Steady-flow device Qin Heat exchanger: Entropy Balance where Case 2 Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 9

Introduction - Objectives: 1. State terminologies and their relations among each other for ideal gases. 2. Write the ideal gas equation in terms of the universal gas constant and in terms the Boltzmann constant. 3. Derive and obtain the relationship between pressure and root mean square speed of molecules. 4. Obtain the relationship of rms speed and gas temperature Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 10

New Way of Looking at Gases Microscopic Variables ØClassical Thermodynamics v. Properties are macroscopic measurables: P, V, T, U v. No inclusion of atomic behaviour v. Did not discuss about the origin of P, T or explain V. T = 30 C P = 4. 246 k. Pa H 2 O: Sat. liquid Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 11

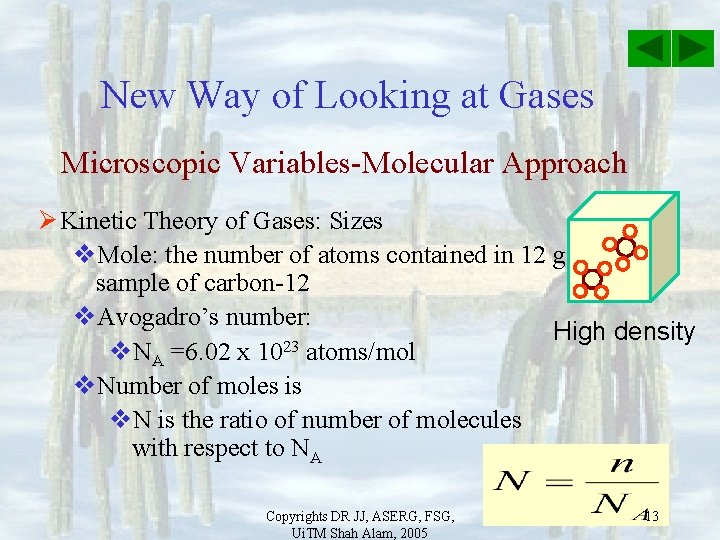
New Way of Looking at Gases Microscopic Variables-Molecular Approach Ø Kinetic Theory of Gases v. Pressure exerted by gas related to molecules colliding with walls v. T and U related to kinetic energies of High density molecules v. V filled by gas relate to freedom of motion of molecules. Ø Must look at same number of molecules when measure size of samples Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 12

New Way of Looking at Gases Microscopic Variables-Molecular Approach Ø Kinetic Theory of Gases: Sizes v. Mole: the number of atoms contained in 12 g sample of carbon-12 v. Avogadro’s number: High density 23 v. NA =6. 02 x 10 atoms/mol v. Number of moles is v. N is the ratio of number of molecules with respect to NA Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 13

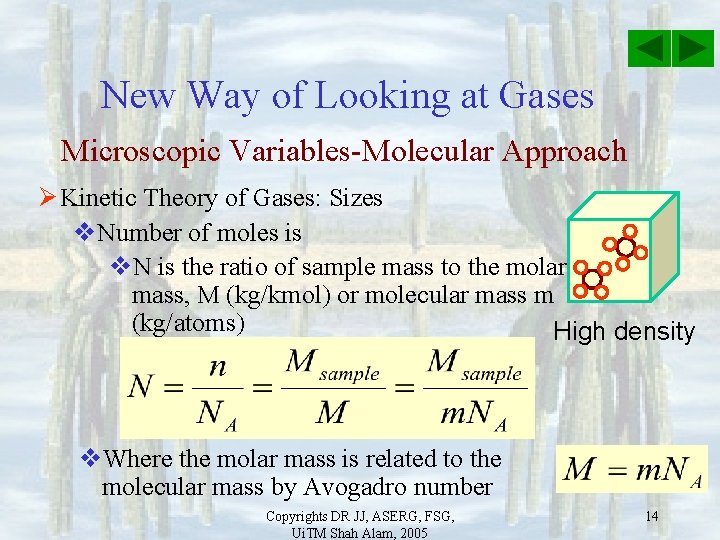
New Way of Looking at Gases Microscopic Variables-Molecular Approach Ø Kinetic Theory of Gases: Sizes v. Number of moles is v. N is the ratio of sample mass to the molar mass, M (kg/kmol) or molecular mass m (kg/atoms) High density v. Where the molar mass is related to the molecular mass by Avogadro number Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 14

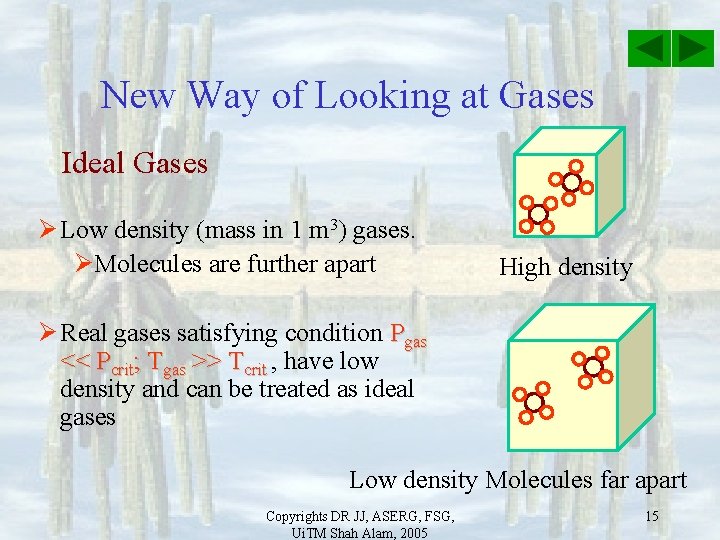
New Way of Looking at Gases Ideal Gases Ø Low density (mass in 1 m 3) gases. ØMolecules are further apart High density Ø Real gases satisfying condition Pgas << Pcrit; Tgas >> Tcrit , have low density and can be treated as ideal gases Low density Molecules far apart Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 15

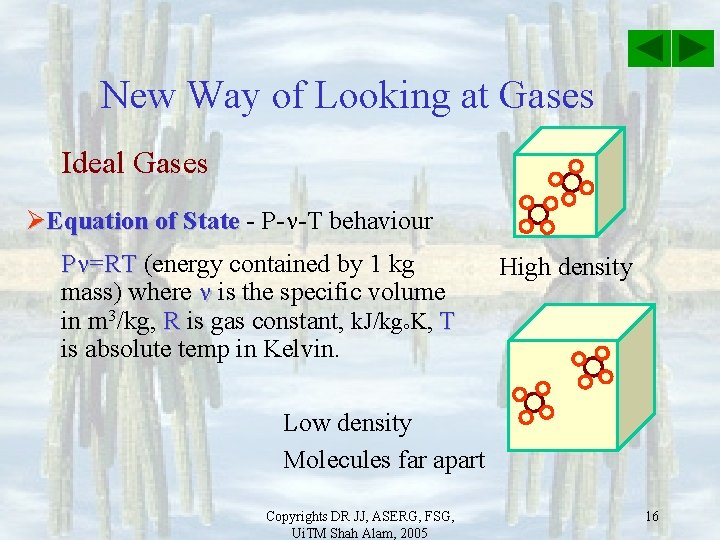
New Way of Looking at Gases Ideal Gases ØEquation of State - P- -T behaviour P =RT (energy contained by 1 kg mass) where is the specific volume in m 3/kg, R is gas constant, k. J/kg K, T is absolute temp in Kelvin. High density Low density Molecules far apart Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 16

New Way of Looking at Gases Ideal Gases ØEquation of State - P- -T behaviour Ø P =RT , since = V/Msam then, P(V/ Msam)=RT. So, PV=Msam. RT, RT in k. Pa m 3=k. J. Ø Total energy of a system. High density Low density Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 17

New Way of Looking at Gases Ideal Gases ØEquation of State - P- -T behaviour Ø PV =Msam. RT =NMRT=N(MR)T But Ru=MR. =MR Hence, can also write PV = NRu. T where Ø N is no of kilomoles, kmol, Ø M is molar mass in kg/kmole , Ø R is a gas constant and Ø Ru is universal gas constant; Ru=MR= 8. 314 k. J/kmol K Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 High density Low density 18

New Way of Looking at Gases Ideal Gases ØEquation of State - P- -T behaviour Ø PV =NRu. T =Nk. NAT=(n/NA)(k. NA)T. Hence, can also write PV = nk. T where Ø N is no of kilomoles, kmol, Ø n is no of molecules, Ø k is Boltzmann constant; Ø Ru = 8. 314 k. J/kmol K = k. NA High density Ø k = Ru / NA = 1. 38 x 10 -23 J/K Low density Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 19

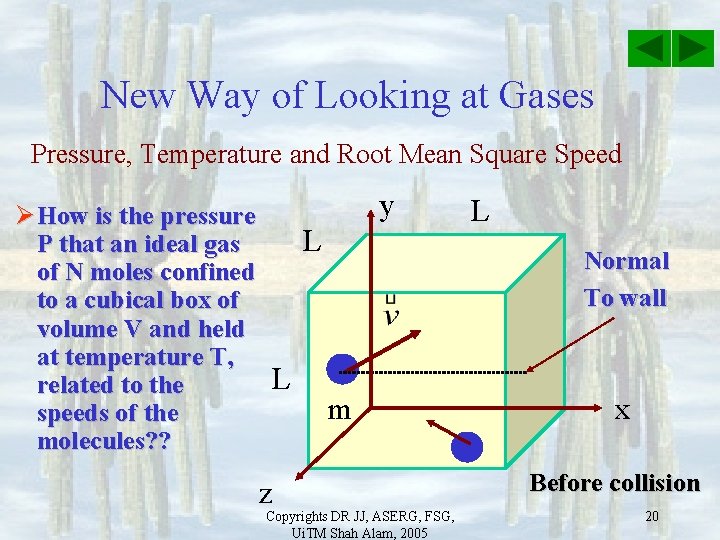
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø How is the pressure P that an ideal gas of N moles confined to a cubical box of volume V and held at temperature T, related to the speeds of the molecules? ? y L L z L Normal To wall m Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 x Before collision 20

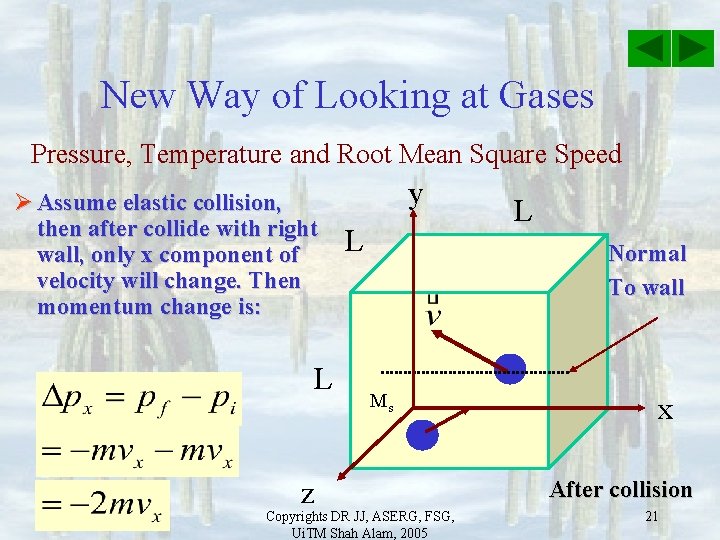
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø Assume elastic collision, then after collide with right wall, only x component of velocity will change. Then momentum change is: L z y L L Normal To wall Ms Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 x After collision 21

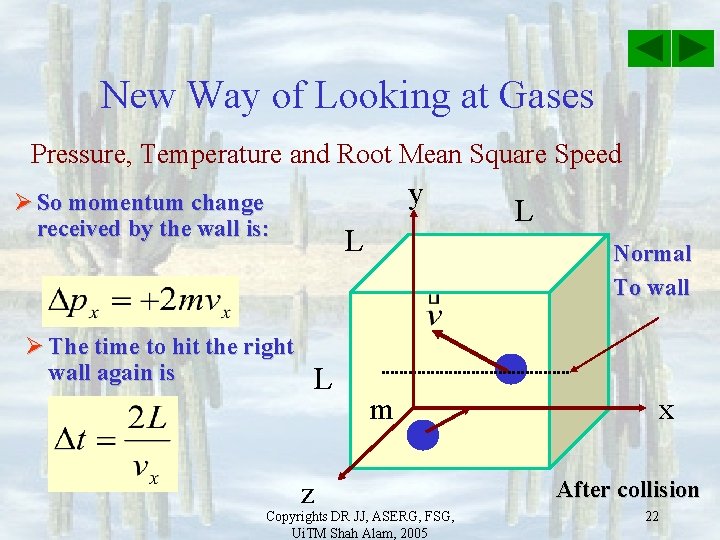
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed y Ø So momentum change received by the wall is: Ø The time to hit the right wall again is L L z L Normal To wall m Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 x After collision 22

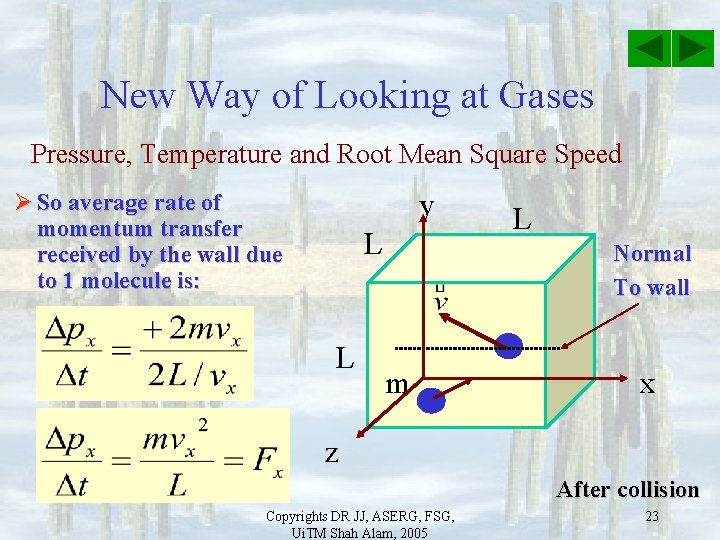
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed y Ø So average rate of momentum transfer received by the wall due to 1 molecule is: L L L Normal To wall m x z After collision Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 23

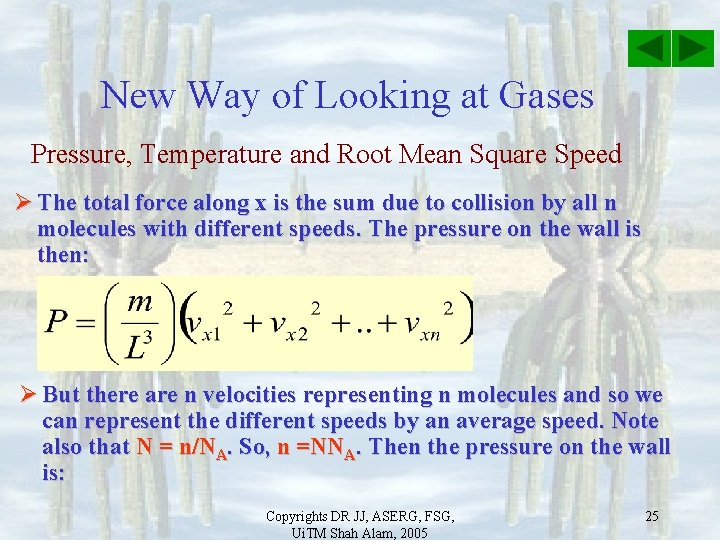
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø The total force along x is the sum due to collision by all N molecules with different speeds. The pressure on the wall is the force exerted for each unit area and is then: Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 24

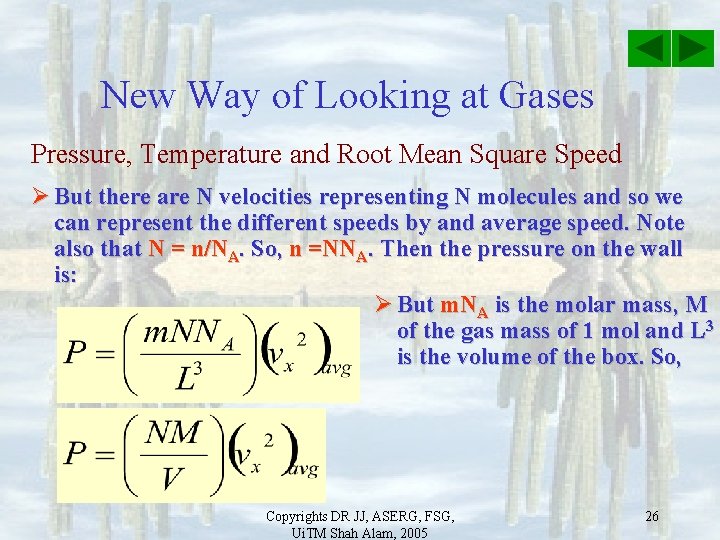
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø The total force along x is the sum due to collision by all n molecules with different speeds. The pressure on the wall is then: Ø But there are n velocities representing n molecules and so we can represent the different speeds by an average speed. Note also that N = n/NA. So, n =NNA. Then the pressure on the wall is: Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 25

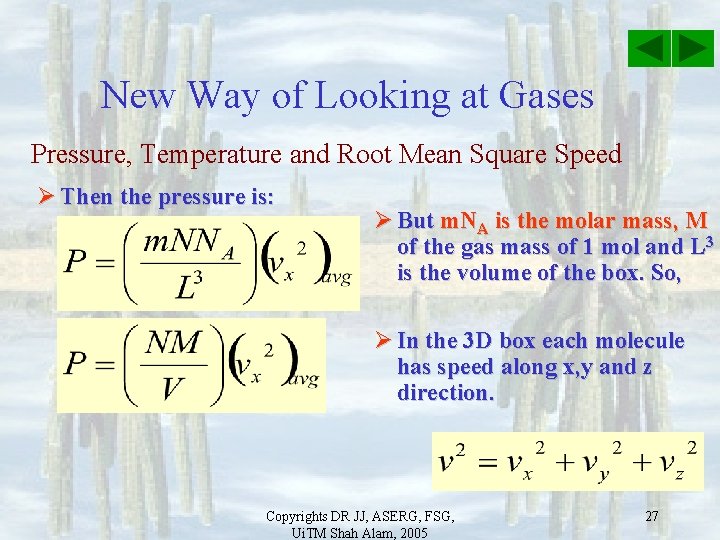
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø But there are N velocities representing N molecules and so we can represent the different speeds by and average speed. Note also that N = n/NA. So, n =NNA. Then the pressure on the wall is: Ø But m. NA is the molar mass, M of the gas mass of 1 mol and L 3 is the volume of the box. So, Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 26

New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø Then the pressure is: Ø But m. NA is the molar mass, M of the gas mass of 1 mol and L 3 is the volume of the box. So, Ø In the 3 D box each molecule has speed along x, y and z direction. Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 27

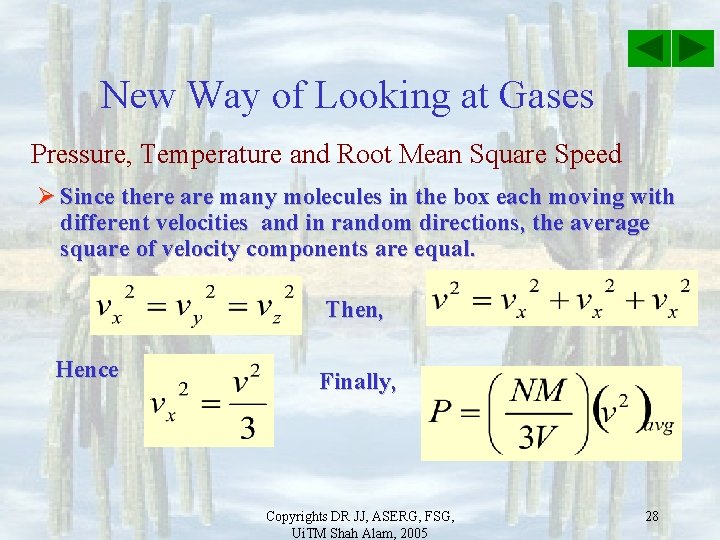
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø Since there are many molecules in the box each moving with different velocities and in random directions, the average square of velocity components are equal. Then, Hence Finally, Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 28

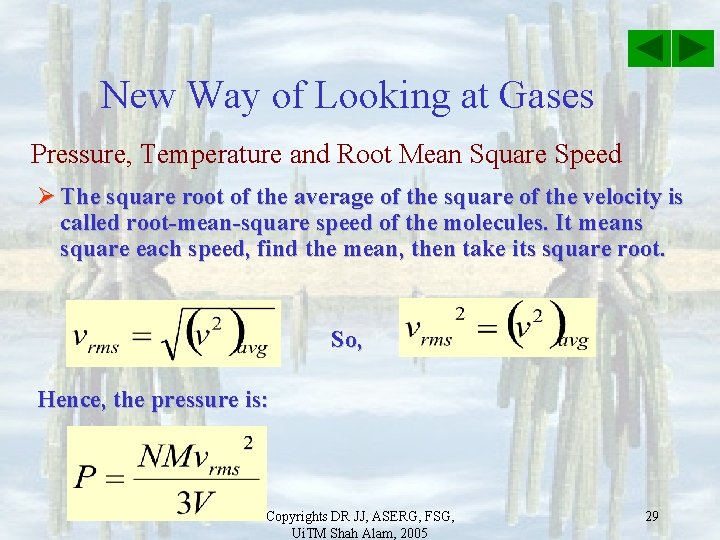
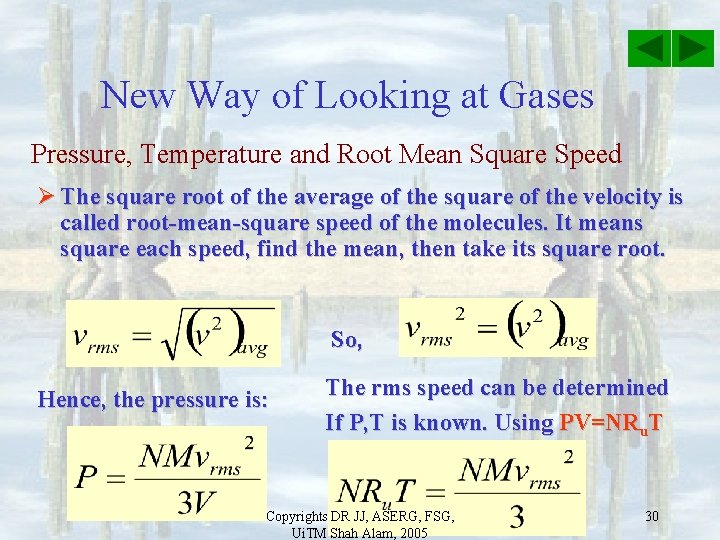
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø The square root of the average of the square of the velocity is called root-mean-square speed of the molecules. It means square each speed, find the mean, then take its square root. So, Hence, the pressure is: Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 29

New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Ø The square root of the average of the square of the velocity is called root-mean-square speed of the molecules. It means square each speed, find the mean, then take its square root. So, Hence, the pressure is: The rms speed can be determined If P, T is known. Using PV=NRu. T Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 30

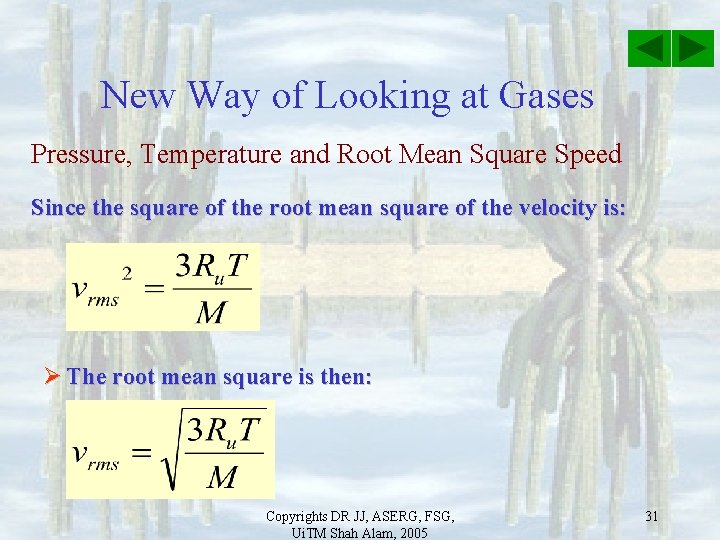
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Since the square of the root mean square of the velocity is: Ø The root mean square is then: Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 31

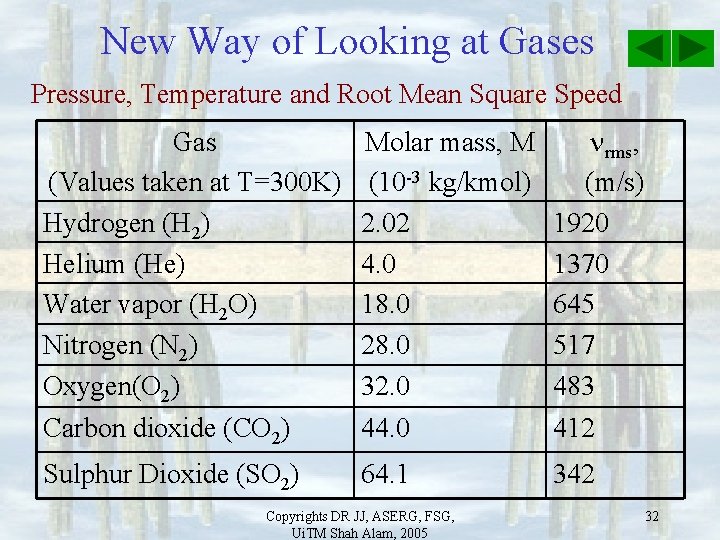
New Way of Looking at Gases Pressure, Temperature and Root Mean Square Speed Gas (Values taken at T=300 K) Hydrogen (H 2) Helium (He) Water vapor (H 2 O) Nitrogen (N 2) Oxygen(O 2) Carbon dioxide (CO 2) Molar mass, M (10 -3 kg/kmol) 2. 02 4. 0 18. 0 28. 0 32. 0 44. 0 rms, (m/s) 1920 1370 645 517 483 412 Sulphur Dioxide (SO 2) 64. 1 342 Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 32

New Way of Looking at Gases Temperature-Translational kinetic Energy Ø Consider a molecule in the box which are colliding with other molecules and changes speed after collision. It moves with translational kinetic energy at any instant Ø But the average translational kinetic energy is over a period of time is: Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 33

New Way of Looking at Gases Temperature-Translational kinetic Energy Ø Substitute the rms speed in terms of T, then: Ø Note that the molar mass M=m. NA. Note also that Ru = k. NA. Hence the average translational kinetic energy is: Regardless of mass , all ideal gas molecules at temperature T have the same avg. translational KE. Copyrights DR JJ, ASERG, FSG, Ui. TM Shah Alam, 2005 34