The Use of Non Profit Industrial Consortia to






![Why Industrial Biomedical Consortia [IBCs]? • Fill a research gap, not being effectively filled Why Industrial Biomedical Consortia [IBCs]? • Fill a research gap, not being effectively filled](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-8.jpg)
![Common Characteristics (TSC/SAEC) • Defined project finite timeframe • Non-Profit [501 c 3] > Common Characteristics (TSC/SAEC) • Defined project finite timeframe • Non-Profit [501 c 3] >](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-10.jpg)


![i. SAEC Organization and Committees BOD Genotyping Core [Expression Analysis, Inc] Scientific/Clinical SMC SAE i. SAEC Organization and Committees BOD Genotyping Core [Expression Analysis, Inc] Scientific/Clinical SMC SAE](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-16.jpg)





![Learning #3 Develop a high-quality plan [before recruiting membership] • Well organized “formation phase” Learning #3 Develop a high-quality plan [before recruiting membership] • Well organized “formation phase”](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-24.jpg)








![Acknowledgments SMC n Ann Daly & DILIGEN [Newcastle] n Mariam Molokhia & EUDRAGENE [London] Acknowledgments SMC n Ann Daly & DILIGEN [Newcastle] n Mariam Molokhia & EUDRAGENE [London]](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-33.jpg)


- Slides: 35

The Use of Non Profit, Industrial Consortia to Improve Pharmaceutical Industry R & D “Lessons Learned” Wellcome Trust’s “Pre-Competitive Boundaries and Open Innovation in Drug Discovery and Development” Meeting Arthur Holden Founder, Chairman, and CEO

Introduction • Executive not scientist • 30 years of leadership experience – commercial & non-profit consortia; start-ups to mid size to large corporations – – Baxter International (1984 -1994) Celsis International (1994 -1998) First Genetic Trust (2000 -2006) Illumina (2006 -2007) • Formed and managed numerous large-scale consortia – The SNP Consortium, Ltd. (1999 -2002) – The International SAE Consortium, Ltd. (2007 - 2012) – Supported many others (MSC, Hap. Map, etc. ) 2

My Comments • Consortia Context – Model – TSC – SAEC • Some Lessons Learned 3

Context

“Pharmaceuticals” Context § Declining Productivity & novel therapeutics § Increasing Costs & Competition at “Market Entry” § “Block Buster” mentality softening § Scale “Innovation” ? ? ? § > Healthcare pricing pressures in all core markets “pay for performance” paradigm of the future § End of “biotech risk subsidy” by the public markets etc, etc. Increasing R & D productivity is critical. 5

“R & D Sourcing Options” External Commercial Partnerships In-house Operations External Academic/Gov. Relationships R&D Consortia 6

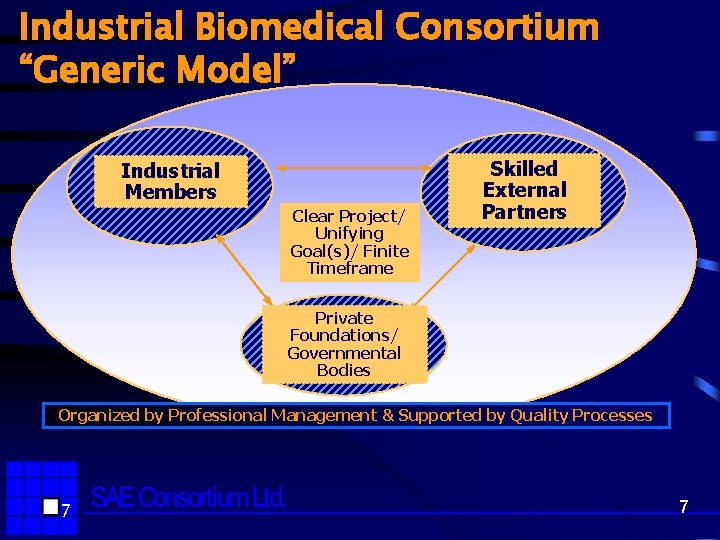
Industrial Biomedical Consortium “Generic Model” Industrial Members Clear Project/ Unifying Goal(s)/ Finite Timeframe Skilled External Partners Private Foundations/ Governmental Bodies Organized by Professional Management & Supported by Quality Processes 7 7
![Why Industrial Biomedical Consortia IBCs Fill a research gap not being effectively filled Why Industrial Biomedical Consortia [IBCs]? • Fill a research gap, not being effectively filled](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-8.jpg)
Why Industrial Biomedical Consortia [IBCs]? • Fill a research gap, not being effectively filled by existing players [government or private] • Establish common/often risky “research platforms”, which help all researchers, while meeting specific industrial scientific requirements [pre-competitive/pro-competitive] • Establish industry standards • Reduce legal/IP barriers • Gain greater efficiency/effectiveness (scale) via pooling of talents and resources across industry 8

Two Consortia The SNP Consortium 1999 -2002 The SAE Consortium 2007 -2012 9
![Common Characteristics TSCSAEC Defined project finite timeframe NonProfit 501 c 3 Common Characteristics (TSC/SAEC) • Defined project finite timeframe • Non-Profit [501 c 3] >](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-10.jpg)
Common Characteristics (TSC/SAEC) • Defined project finite timeframe • Non-Profit [501 c 3] > “public benefit orientation” – Open data access – No IP constraints • “Fixed, uniform” membership contributions • Designed for “leverage” and “time to result” • Collaboration with government (if advantageous to results) • Active (Committee) Participation and Investment by Members • “Governance by majority” 1 member/1 vote 10

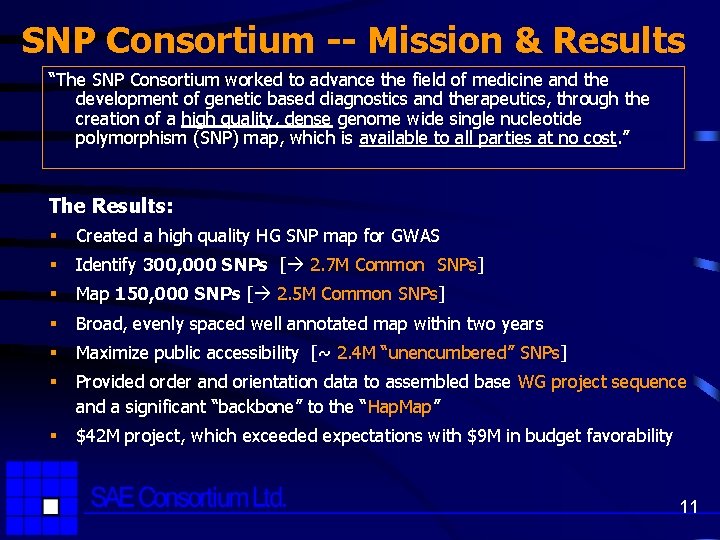
SNP Consortium -- Mission & Results “The SNP Consortium worked to advance the field of medicine and the development of genetic based diagnostics and therapeutics, through the creation of a high quality, dense genome wide single nucleotide polymorphism (SNP) map, which is available to all parties at no cost. ” The Results: § Created a high quality HG SNP map for GWAS § Identify 300, 000 SNPs [ 2. 7 M Common SNPs] § Map 150, 000 SNPs [ 2. 5 M Common SNPs] § Broad, evenly spaced well annotated map within two years § Maximize public accessibility [~ 2. 4 M “unencumbered” SNPs] § Provided order and orientation data to assembled base WG project sequence and a significant “backbone” to the “Hap. Map” § $42 M project, which exceeded expectations with $9 M in budget favorability 11

Drivers of the SNP Consortium ü Industry standard SNP map strong industry support ü Universal access to base genomic/SNP data public domain (IP considerations) ü Facilitate pharmaco-genomics / genetics association, linkage & haplotyping studies ü Economics: § Favorable economy of scale>>cost & risk sharing § Favorable cost to access high quality SNP datasets 12

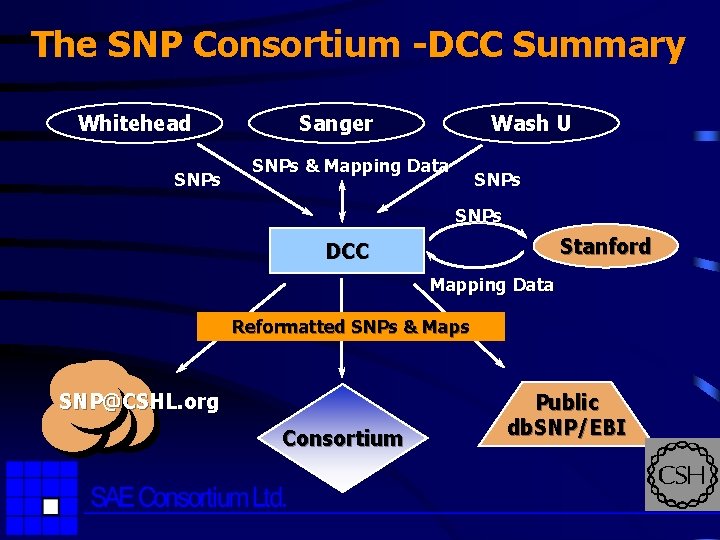
The SNP Consortium -DCC Summary Whitehead SNPs Sanger Wash U SNPs & Mapping Data SNPs Stanford DCC Mapping Data Reformatted SNPs & Maps SNP@CSHL. org Consortium Public db. SNP/EBI

i. SAEC -- Mission & Results “The i. SAEC will identify and validate DNA-variants useful in predicting the risk of drug induced serious adverse events. ” The Results (to date): § Initial GWAS for Drug Induced Serious Skin Rash identifying marker(s) of potential significance § Assembled 2 nd largest DILI cohort, merged with DILIN, completed initial GWAS identifying numerous significant marker(s) within/across drugs § Initial GWAS for Drug Induced PQT/Td. P, merged with Le. Dueq Network, and identified marker(s) of potential significance § Completed four public data releases (clinical and GWAS data) with FDA § > 200 genetic markers associated with DILI and SSR in the public domain. § Favorable to budget in phase by $2 M § Organized Phase 2 focused on “drug induced immunologic SAEs” associated with specific drugs and ethnicities, and forming novel SAE research channels vital to drug-specific SAE research. 14

Drivers of the i. SAEC ü Need to improve new product (safety) productivity ü Scale required to execute safety pharmacogenetics (top R & D management priority) common markers in public domain ü Need to standardize “SAE phenotypes” ü Need to develop new and innovative methods to source cases and controls ü Pooled technology assessment & risk (e. g. GWAS, WG sequencing) ü FDA’s Industrial Advisory Board recommendation ü $ resources required (poor public funding, given the health impacts) 15
![i SAEC Organization and Committees BOD Genotyping Core Expression Analysis Inc ScientificClinical SMC SAE i. SAEC Organization and Committees BOD Genotyping Core [Expression Analysis, Inc] Scientific/Clinical SMC SAE](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-16.jpg)
i. SAEC Organization and Committees BOD Genotyping Core [Expression Analysis, Inc] Scientific/Clinical SMC SAE [s] Bioinformatics Core [Columbia University] CEO Legal/IP PR/ Comm. Data Anal. Research Collaborators [Academic & Commercial] Outsourced Service Suppliers [Legal & PR] 16

i. SAEC Web Site http: //www. saeconsortium. org 17

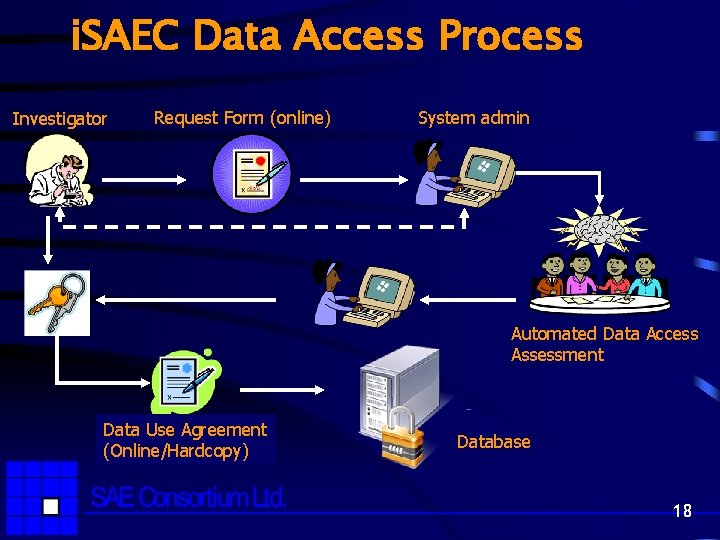
i. SAEC Data Access Process Investigator Request Form (online) System admin Automated Data Access Assessment Data Use Agreement (Online/Hardcopy) Database 18

Lessons Learned

10 Lessons Learned (Success Factors) 1. A clear unifying objective is a must. 2. Ensure the effort is both “pre-competitive” and “pro-competitive. ” 3. Have a quality “draft” operating concept/plan before recruiting members [“one shot rule”]. 4. Establish a uniform membership requirements & development strategy with potential members. 5. Establish dedicated management early in the effort /don’t reinvent the wheel. 20

10 Success Factors cont. 6. Organize well defined committees with high quality, dedicated leaders 7. Outsource…outsource… outsource to the best external advisors/ investigators via “performance based” contracts 8. Generate results that exceed expectations 9. Make it fun and say “thank you” in meaningful ways, and…. 10. Know when to fold … begin with an end in mind! 21

Learning #1 Have a Clear, Quality Objective which Unifies the Membership […Full Genome Representative SNP Map… Safety related markers in the public domain] • Needed Science! • Easily Understood • Measurable • Focused vs General • All essential for member recruitment 22

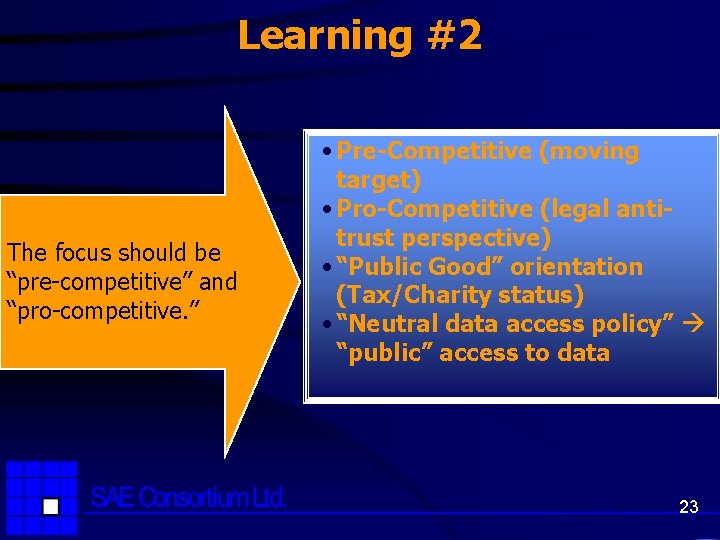
Learning #2 The focus should be “pre-competitive” and “pro-competitive. ” • Pre-Competitive (moving target) • Pro-Competitive (legal antitrust perspective) • “Public Good” orientation (Tax/Charity status) • “Neutral data access policy” “public” access to data 23
![Learning 3 Develop a highquality plan before recruiting membership Well organized formation phase Learning #3 Develop a high-quality plan [before recruiting membership] • Well organized “formation phase”](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-24.jpg)
Learning #3 Develop a high-quality plan [before recruiting membership] • Well organized “formation phase” with potential members • Scientific Plan [including necessary feasibility pilots] • Informatics Plan • Legal/IP Policies • Operating Plan/Performance Metrics/Contingencies • Budget [Proactive Risk Factor Mgnt] 24

Learning #4 Establish a Clear Membership Requirement & Development Strategy with potential members • High Priority a Must! • Fixed Dues Structure (Annual Sensitivity [$1 M down to <$. 4 M/year) • Do not vary membership tiers for industrial members • Non industrial members >> same $ terms for voting rights • Member Diversification has pros & cons 25

Learning #5 Establish dedicated management ASAP • Formation phase leadership • Members are investing in both the leader/project • Defining the Organization & Key Processes • Sourcing strategies • Pre-formation negotiations • “Closing” Membership • Communication is Key • Everything takes more Effort than Planned 26

Learning #6 Organize clear committees with high quality dedicated leaders • SMC, Legal/IP, & Subcommittees key • Functional/Scientific Leadership is vital • Time Commitment is Greater than Expected • Senior Management Support of Manager Commitment 27

Learning #7 Outsource…outsource… outsource • Increases expertise & skills • Leverage existing infrastructure/investments/skill • Performance based contracts no grants! • Reduces project risk • Increases management flexibility • Careful assessment of the risks of doing the Consortium if Outsourcing is not an Option

Learning #8 Generate results that exceed expectations! • Confirms membership investment creates additional consortia opportunities • “Consortia fatigue” is real major cause is poorly managed/under performing consortia • Winning together is fun … 29

Learning #9 Say “thank you” in meaningful ways and make the consortium a fun experience • Member help is essential • “Recognition starvation” is an epidemic • Everybody likes “special events” … but costeffectiveness is vital in today’s environment 30

Learning #10 Don’t try to make a consortium into something it shouldn't be … know when to fold • Finite objectives within a fixed time frame attractive to members • Consortia are strategic initiatives vs on-going concerns • New Consortia require different leadership • Effective BOD with the appropriate experience & seniority—Key!

Final Caveats • Wellcome Trust involvement and funding important validation to pharmcos • Involve stakeholder governmental bodies (TSC— NIH, i. SAEC—FDA, EMEA, PMDA), without slowing the effort. • Limits to company leverage in these “leaner times” … funding a quality consortium is very hard these days (more freeloaders) • Vital that we learn from best efforts take these into new, important consortia efforts to improve pharma productivity 32
![Acknowledgments SMC n Ann Daly DILIGEN Newcastle n Mariam Molokhia EUDRAGENE London Acknowledgments SMC n Ann Daly & DILIGEN [Newcastle] n Mariam Molokhia & EUDRAGENE [London]](https://slidetodoc.com/presentation_image/3460a9da3b5aac70b6f7ffea1d0b48fa/image-33.jpg)
Acknowledgments SMC n Ann Daly & DILIGEN [Newcastle] n Mariam Molokhia & EUDRAGENE [London] n Matt Nelson [GSK] n Sally John [Pfizer] n Yufeng Shen [Columbia] n Itsik Pe’er [Columbia] n Aris Floratos [Columbia] n Mark Daly [Harvard/Broad] n David Goldstein [Duke] n Eric Lai [ex-GSK] n Donald Halbert [Abbott] n Joe Walker [D-S] n Nadine Cohen, Quingqin Serena Li, & Adrian Thomas [J&J] n Joanne Meyer & Steve Lewitzky [Novartis] n Klaus Lindpaintner & Karen Wilcock [Roche] n Steven Kovacs [Sanofi-Aventis] n Leonardo Sahelijo [Takeda] n Ted Burczynski & Maha Karnoub [Wyeth] n Robert O'Neill & Steve Wilson [FDA] n Andrea Califano [Columbia] n Allen Roses [Duke] n John Senior [FDA] 33 Members n Brian Spear [Abbott] n Rick Scheyer [Daiichi Sankyo] n Lon Cardon [GSK] n Nadine Cohen [J&J] n Joanne Meyer [Novartis] n Aidan Power [Pfizer] n Klaus Lindpaintner [Roche] n Robert Dix [Sanofi-Aventis] n Leonardo Sahelijo [Takeda] n Michael Burczynski [Wyeth] n Janet Woodcock [FDA] n Sha. Avhree Buckman [FDA] n Michael Dunn [Wellcome Trust]

Appendix Slides

Industrial Consortia Some Operational Tenants • Unifying objective industry and public good [501 c 3] • Focused projects, with clear objectives & strong operational management • Strong “quality” and “time to result” orientation • Clear and uniform “membership requirements” • Extensive leverage of members’ skills via well organized sub-committees • Strive to collaborate with the best quality external advisors & investigators on an international basis • Public release of data after appropriate IP management actions to ensure “openness” 35
 Mutual service consortia example
Mutual service consortia example Mutual service consortia
Mutual service consortia Mutual service consortia
Mutual service consortia Profit maximization
Profit maximization Economic profit vs accounting profit
Economic profit vs accounting profit Post acquisition profit is which profit
Post acquisition profit is which profit Pour cet immense bonheur paroles
Pour cet immense bonheur paroles Non profit organization mission
Non profit organization mission Private limited company definition ib business
Private limited company definition ib business Non profit corporate structure
Non profit corporate structure Gloria misfeldt
Gloria misfeldt Non profit organization definition
Non profit organization definition Non profit air ambulance
Non profit air ambulance Is sentara non profit
Is sentara non profit Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Glasgow thang điểm
Glasgow thang điểm Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8