The States of Matter There are 4 states






- Slides: 24

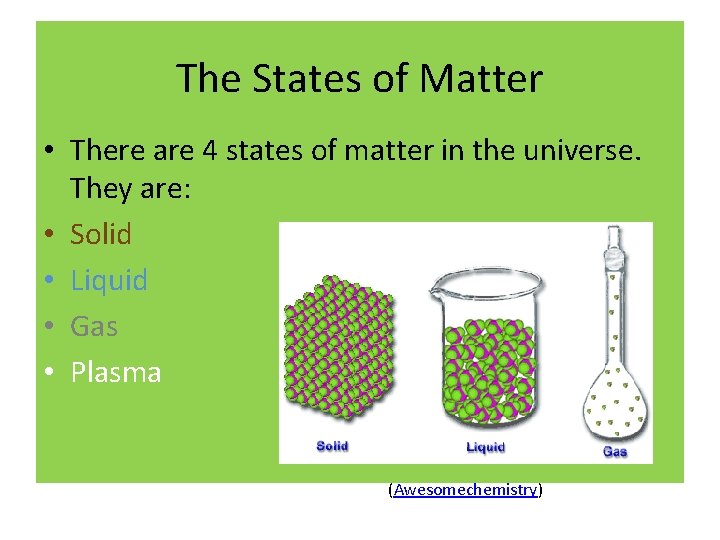
The States of Matter • There are 4 states of matter in the universe. They are: • Solid • Liquid • Gas • Plasma (Awesomechemistry)

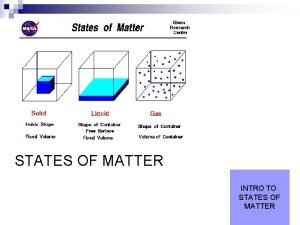
Solids Out of the states of matter, solids have the lowest amount energy in their molecules. This causes the molecules to pack closely together. Solids have a definite shape (meaning that they would not take on the size of any container they may be put in). 100 Kg

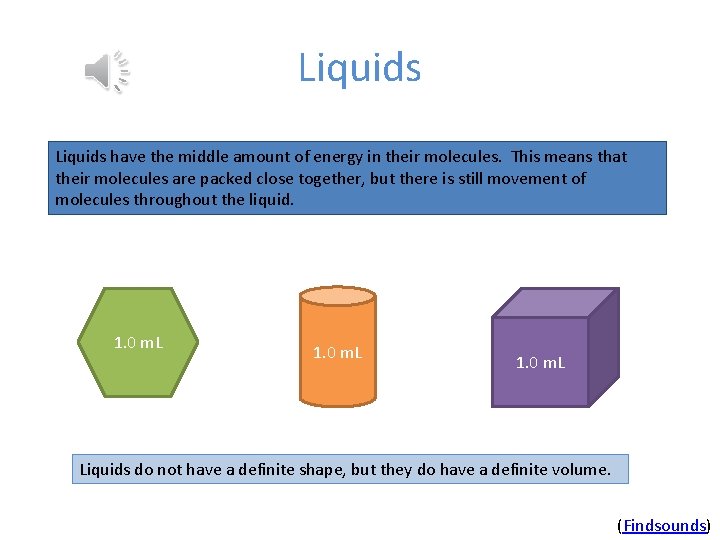
Liquids have the middle amount of energy in their molecules. This means that their molecules are packed close together, but there is still movement of molecules throughout the liquid. 1. 0 m. L Liquids do not have a definite shape, but they do have a definite volume. (Findsounds)

Gases have the highest amount of energy in their molecules. The molecules are unbound to each other, thus leaving the molecules free to move about. Gases have neither a definite shape nor a *definite volume. *Temperature and pressure can affect a gas’s volume.

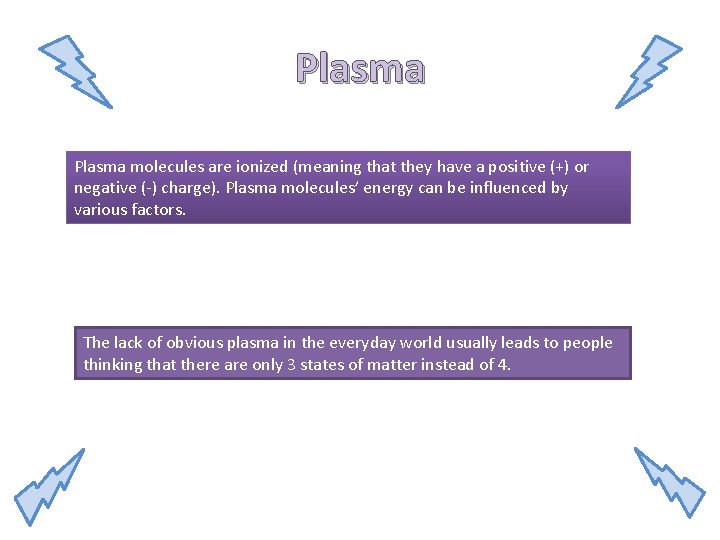
Plasma molecules are ionized (meaning that they have a positive (+) or negative (-) charge). Plasma molecules’ energy can be influenced by various factors. The lack of obvious plasma in the everyday world usually leads to people thinking that there are only 3 states of matter instead of 4.

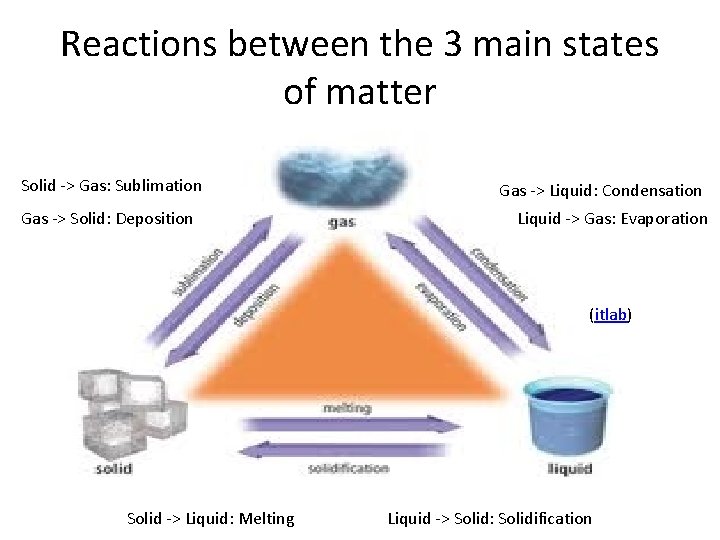
Reactions between the 3 main states of matter Solid -> Gas: Sublimation Gas -> Solid: Deposition Gas -> Liquid: Condensation Liquid -> Gas: Evaporation (itlab) Solid -> Liquid: Melting Liquid -> Solid: Solidification

Quiz Time! Multiple Choice. Click “answer” next to the best choice for the given question. Good luck!

A solid’s molecules have: Low amounts of energy (the molecules are packed together) Answer High amounts of energy (the molecules move around unbound and completely free) Answer

Correct! Next Question

Sorry, incorrect. A high amount of energy, where molecules are unbound and move freely, would be used to describe a gas Next Question

What state of matter has a set volume but no set shape? a. b. c. d. Solid Answer Liquid Answer Gas Answer Plasma Answer

Correct! Next Question

Sorry, incorrect. The state of matter that has a set volume but no set shape describes a liquid Next Question

Which state of matter is commonly unknown about? a. b. c. d. Solid Answer Liquid Answer Gas Answer Plasma Answer

Correct! Next Question

Sorry, incorrect. The most commonly known states of matter are: solid, liquid, and gas. The most uncommonly known state would be plasma. Next Question

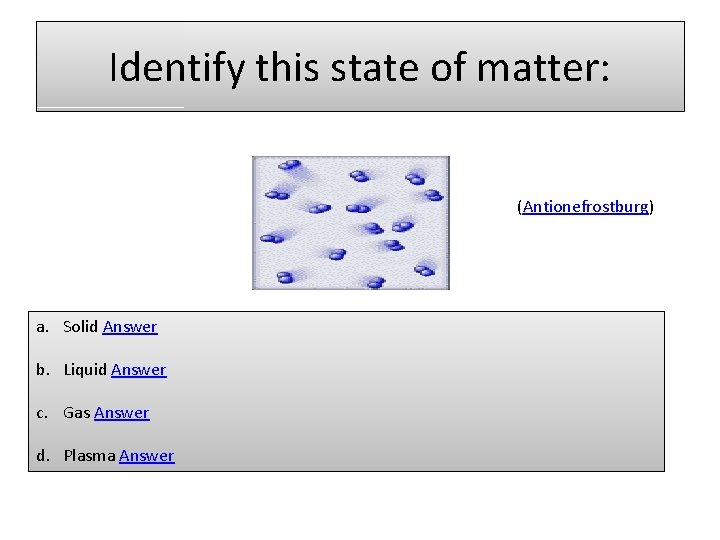
Identify this state of matter: (Antionefrostburg) a. Solid Answer b. Liquid Answer c. Gas Answer d. Plasma Answer

Correct! Next Question

Sorry, incorrect. The space between the particles and the lines of movement would indicate that this is the gas state of matter. Next Question

Sublimation refers to what reaction/change of state. a. b. c. d. Gas -> Solid Answer Liquid -> Gas Answer Solid -> Gas Answer Gas -> Liquid Answer

Correct! End

Sorry, incorrect. Sublimation refers to the process of a solid changing to a gas. An example of this change can be seen in solid CO 2/“Dry Ice. ” End

Thanks for taking the quiz!!! Resources Retake Quiz

Sources Cited • Antionefrostburg: http: //antoine. frostburg. edu/chem/senese/10 1/matter/print-index. shtml • Awesomechemistry: http: //awesomechemistry. com/Period-8. php • Findsounds: http: //www. findsounds. com/ • Itlab: http: //itlab 2. coe. wayne. edu/akayed/file_page s/activities 2. html
 Mikael ferm
Mikael ferm Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter What is the difference between gray and grey
What is the difference between gray and grey Composition of matter section 1
Composition of matter section 1 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers What is grey and white matter
What is grey and white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Dural septa
Dural septa Interconversion of states of matter
Interconversion of states of matter Plasma particle arrangement
Plasma particle arrangement Whats the four states of matter
Whats the four states of matter Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter
States of matter States of matter jeopardy
States of matter jeopardy States of matter
States of matter All matter is in constant motion
All matter is in constant motion Use of heat
Use of heat Solid diagram particles
Solid diagram particles Matter classification flow chart
Matter classification flow chart Mind map on states of matter
Mind map on states of matter Phases of matter foldable
Phases of matter foldable States of matter
States of matter States of matter basics
States of matter basics