THE PURE ROTATIONAL SPECTRUM OF PERFLUOROOCTANONITRILE C 7




- Slides: 20

THE PURE ROTATIONAL SPECTRUM OF PERFLUOROOCTANONITRILE, C 7 F 15 CN, STUDIED USING CAVITY- AND CHIRPED-PULSED FOURIER TRANSFORM MICROWAVE SPECTROSCOPIES. W. C. Bailey, R. Bohn, C. T. Dewberry, G. S. Grubbs II and S. A. Cooke WH 13

1938 – Dr. Roy Plunkett Discovers Polytetrafluoroethene (PTFE)

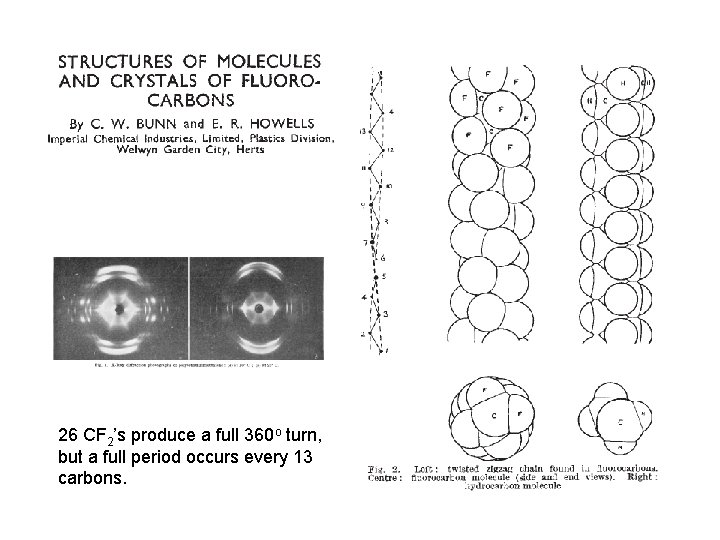
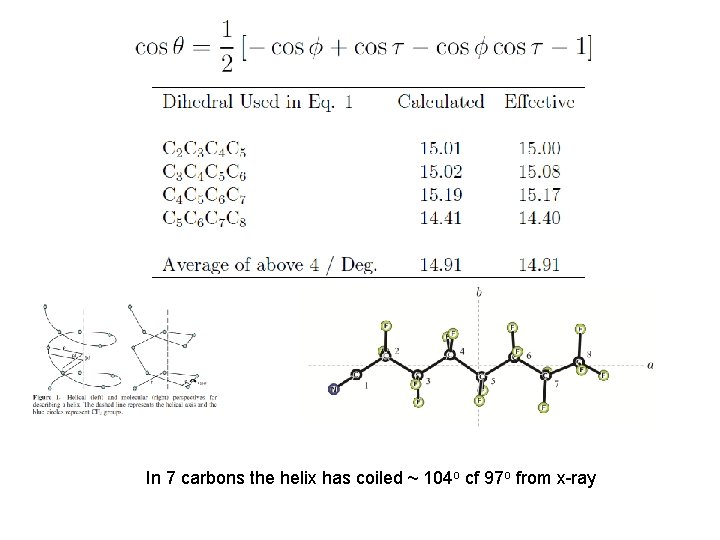
26 CF 2’s produce a full 360 o turn, but a full period occurs every 13 carbons.


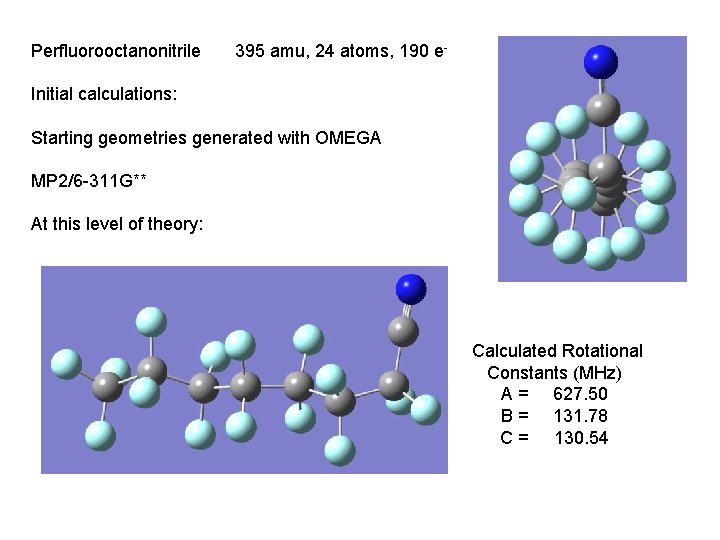
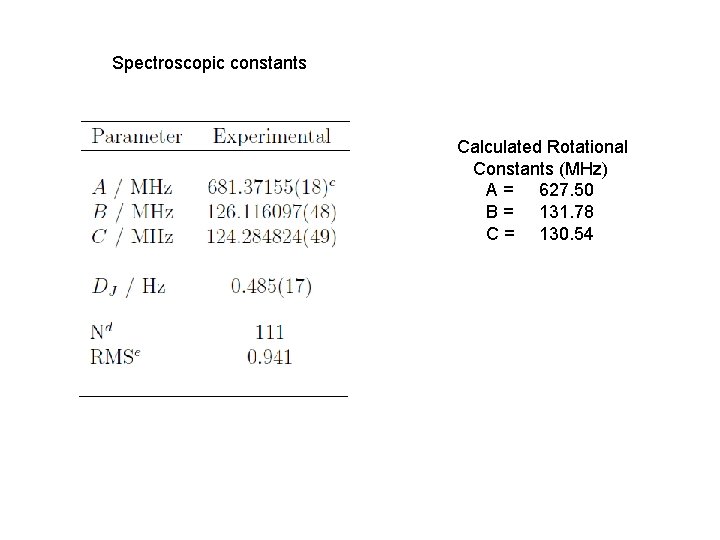
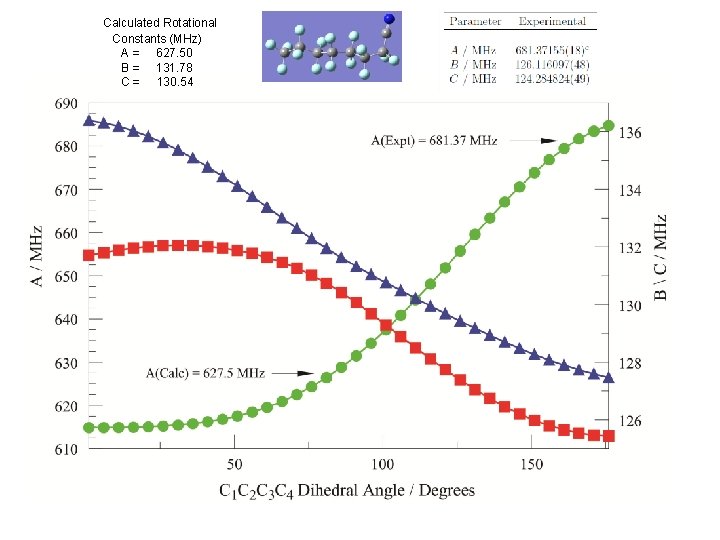
Perfluorooctanonitrile 395 amu, 24 atoms, 190 e- Initial calculations: Starting geometries generated with OMEGA MP 2/6 -311 G** At this level of theory: Calculated Rotational Constants (MHz) A = 627. 50 B = 131. 78 C = 130. 54

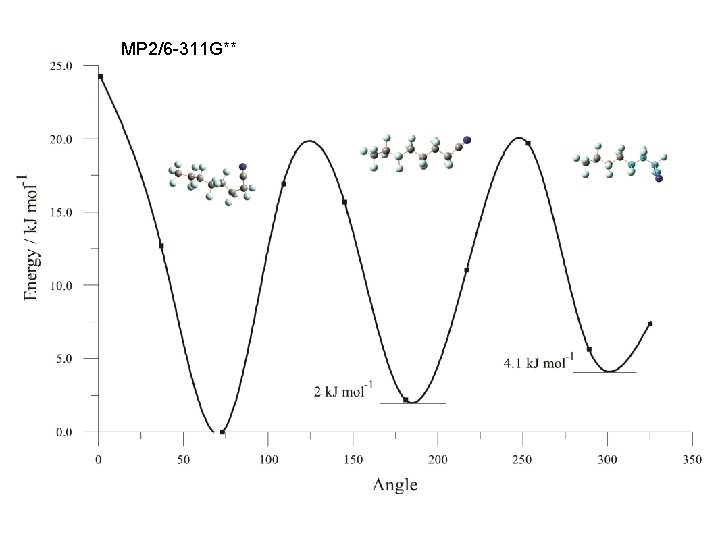
MP 2/6 -311 G**

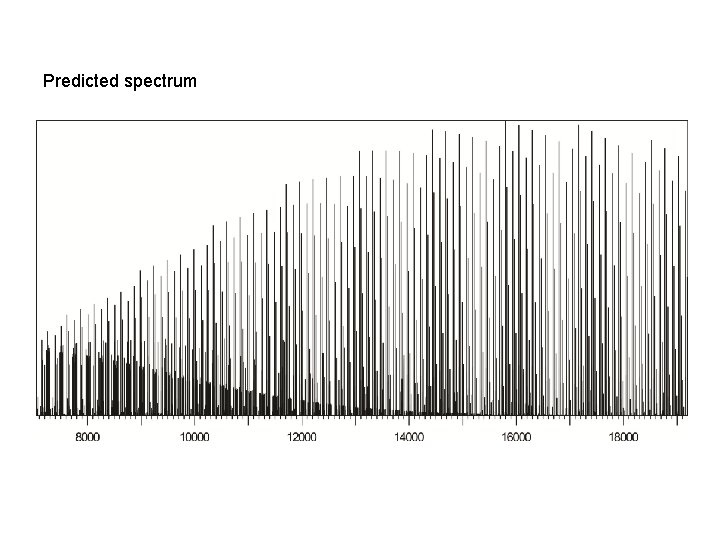
Predicted spectrum

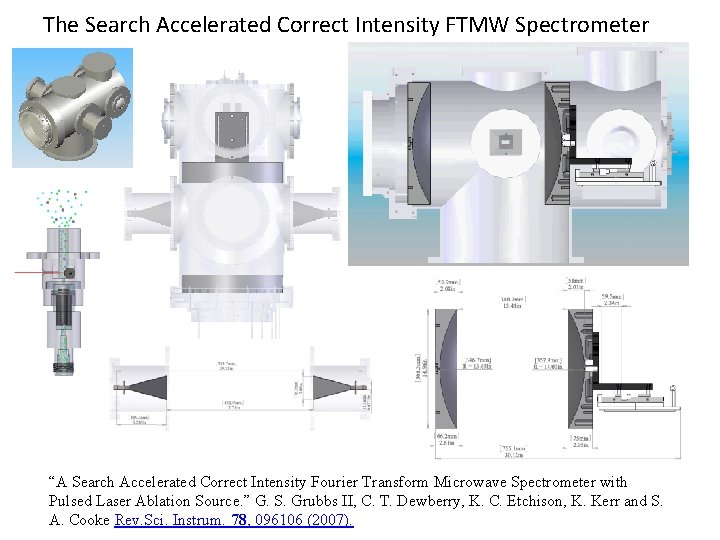
The Search Accelerated Correct Intensity FTMW Spectrometer “A Search Accelerated Correct Intensity Fourier Transform Microwave Spectrometer with Pulsed Laser Ablation Source. ” G. S. Grubbs II, C. T. Dewberry, K. C. Etchison, K. Kerr and S. A. Cooke Rev. Sci. Instrum. 78, 096106 (2007).

Circuits RH 03 Thurs 2. 04 pm Acknowledgements: Brooks Pate Jens-Uwe Grabow

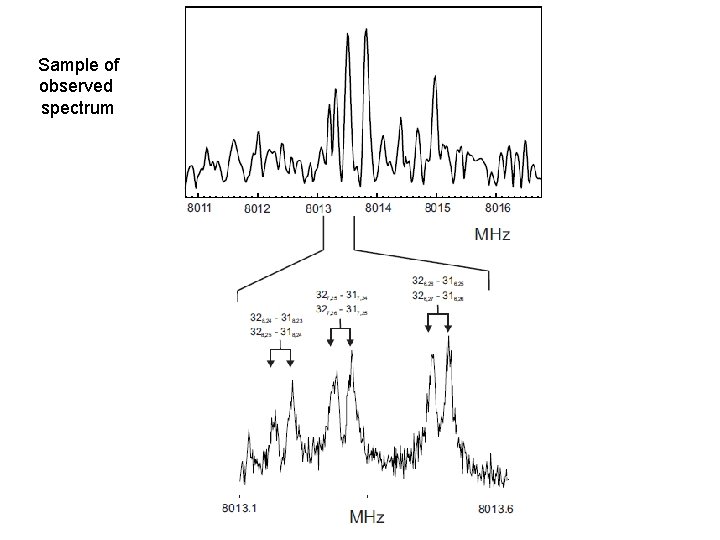
Sample of observed spectrum

Spectroscopic constants Calculated Rotational Constants (MHz) A = 627. 50 B = 131. 78 C = 130. 54

Calculated Rotational Constants (MHz) A = 627. 50 B = 131. 78 C = 130. 54

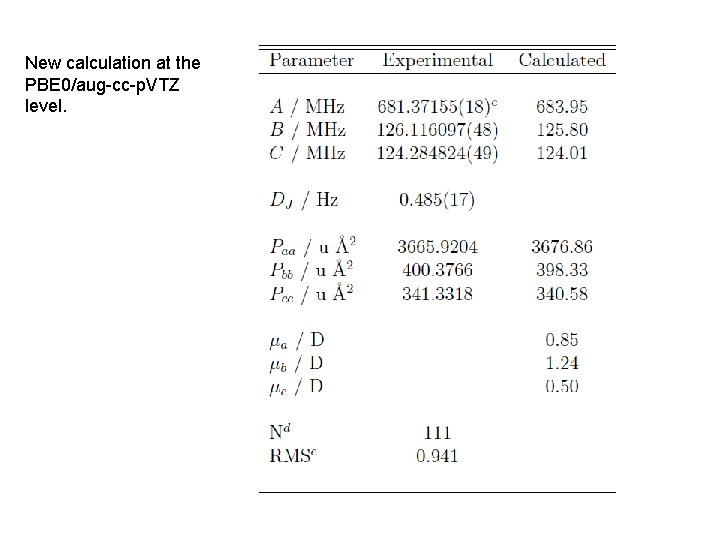
New calculation at the PBE 0/aug-cc-p. VTZ level.

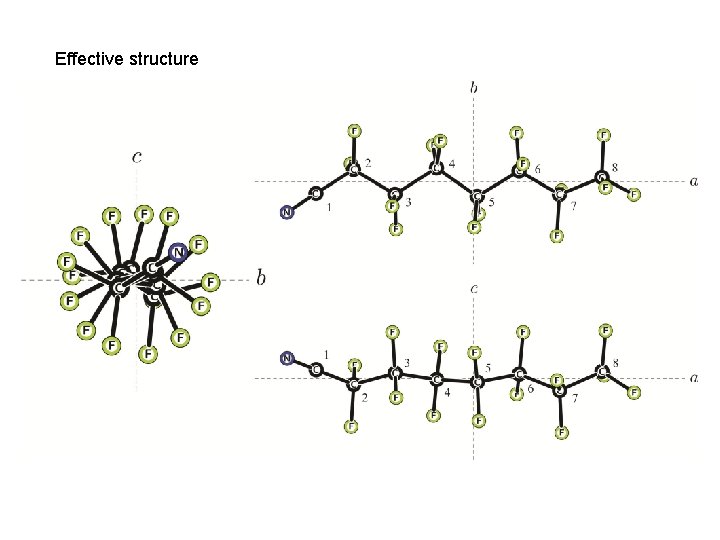
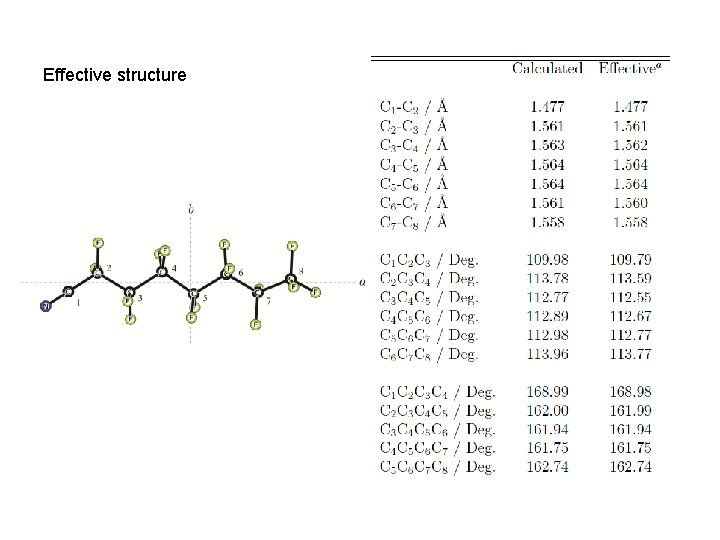
Effective structure

Effective structure

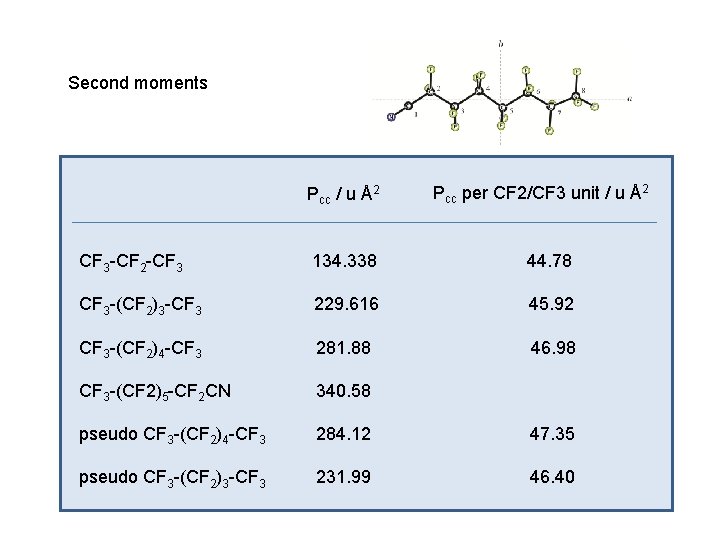
Second moments Pcc / u Å2 Pcc per CF 2/CF 3 unit / u Å2 CF 3 -CF 2 -CF 3 134. 338 44. 78 CF 3 -(CF 2)3 -CF 3 229. 616 45. 92 CF 3 -(CF 2)4 -CF 3 281. 88 46. 98 CF 3 -(CF 2)5 -CF 2 CN 340. 58 pseudo CF 3 -(CF 2)4 -CF 3 284. 12 47. 35 pseudo CF 3 -(CF 2)3 -CF 3 231. 99 46. 40

In 7 carbons the helix has coiled ~ 104 o cf 97 o from x-ray

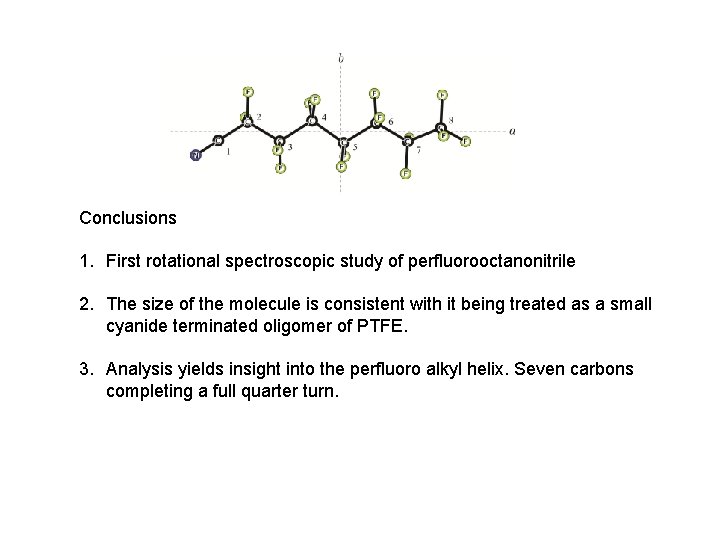
Conclusions 1. First rotational spectroscopic study of perfluorooctanonitrile 2. The size of the molecule is consistent with it being treated as a small cyanide terminated oligomer of PTFE. 3. Analysis yields insight into the perfluoro alkyl helix. Seven carbons completing a full quarter turn.

Acknowledgements • Laboratory Funding from the NSF

• Fourier transform rotational spectroscopy has been used to collect the spectrum of perfluooctanonitrile. The spectrum was weak and only one conformer was observed. The assigned spectrum currently consists of both a- and b-type transitions spanning J = 8 to 40. The rotational constants are small, A = 681. 37155(18) MHz, B = 126. 116097(48) MHz, and C = 124. 284824(49) MHz. The spectroscopic constants together with quantum chemical calculations have been used to identify the structure of the observed conformer. Notably the helical nature of the perfluoro alkyl chain is fully in evidence. Further calculations confirm that the nitrogen quadrupole coupling tensor is such that nitrogen hyperfine splitting will not be observable at the high J transitions recorded in our experiments. Spectroscopic constants and a discussion of the molecular structure will be presented.
 Torque right hand rule
Torque right hand rule Rotational equilibrium and rotational dynamics
Rotational equilibrium and rotational dynamics Absorption spectrum
Absorption spectrum D orbital shape
D orbital shape Pure spectrum of light
Pure spectrum of light Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worms-breton
Tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dot
Dot