Pure rotational spectrum of the nonpolar dimer of






- Slides: 18

Pure rotational spectrum of the “nonpolar” dimer of Formic acid Weixing Li, Luca Evangelisti, Qian Gou, Rolf Meyer, Walther Caminati* Supervisors: Prof. Sonia Melandri Prof. Walther Caminati Università di Bologna

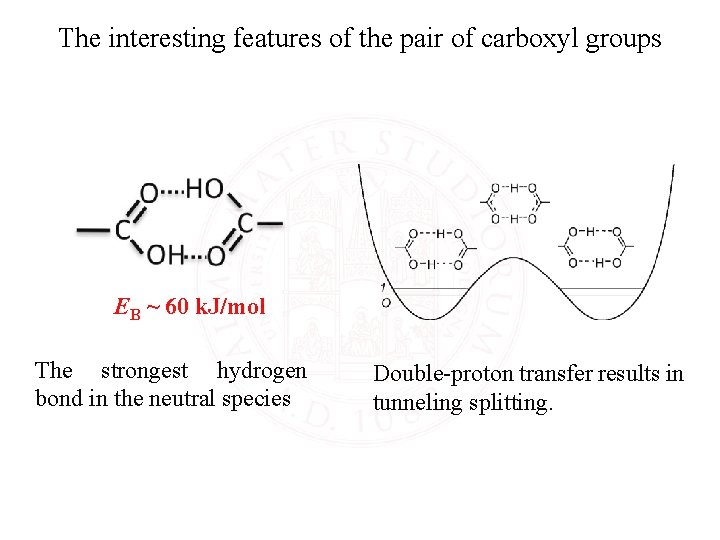
The interesting features of the pair of carboxyl groups EB ~ 60 k. J/mol The strongest hydrogen bond in the neutral species Double-proton transfer results in tunneling splitting.

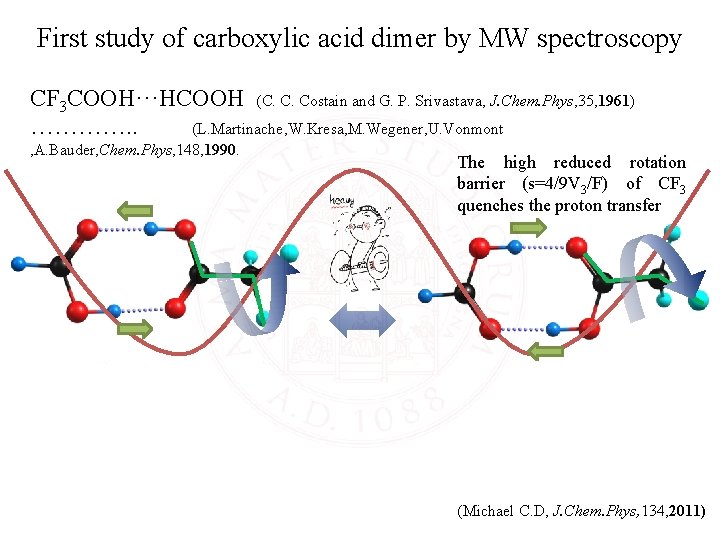
First study of carboxylic acid dimer by MW spectroscopy CF 3 COOH···HCOOH (C. C. Costain and G. P. Srivastava, J. Chem. Phys, 35, 1961) …………. . (L. Martinache, W. Kresa, M. Wegener, U. Vonmont , A. Bauder, Chem. Phys, 148, 1990. The high reduced rotation barrier (s=4/9 V 3/F) of CF 3 quenches the proton transfer The CH 3 group rotation coupling the double-proton transfer complicate the spectroscopic study. (Michael C. D, J. Chem. Phys, 134, 2011)

The pure double protons transfer studied by MW spectroscopy 548. 7 MHz 2442 cm-1 Luca Evangelisti, Walther Caminati, et al. , J. Phys. Chem. Lett. 2012, 3, 3770− 3775

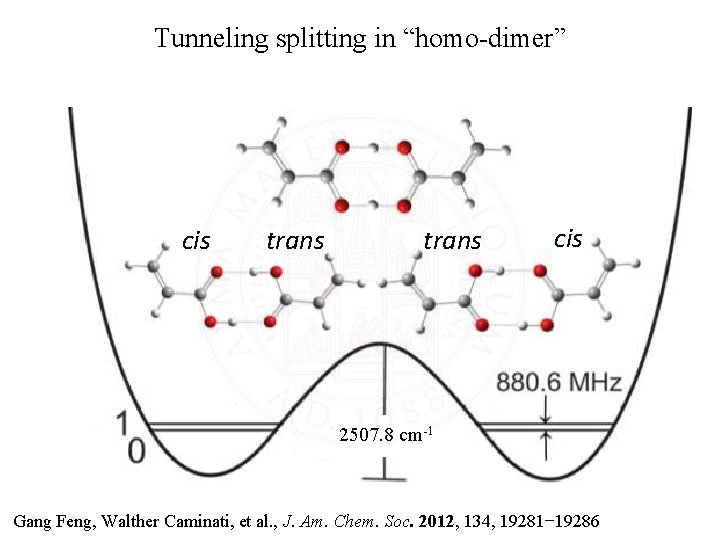
Tunneling splitting in “homo-dimer” cis trans cis 2507. 8 cm-1 Gang Feng, Walther Caminati, et al. , J. Am. Chem. Soc. 2012, 134, 19281− 19286

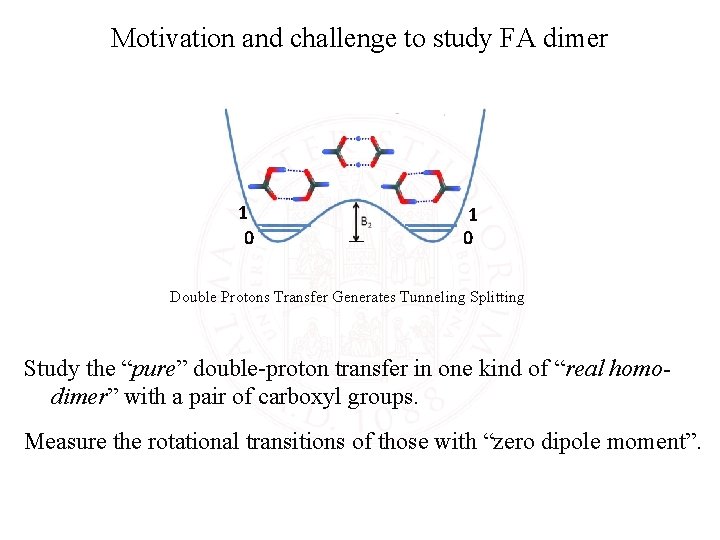
Motivation and challenge to study FA dimer 1 0 Double Protons Transfer Generates Tunneling Splitting Study the “pure” double-proton transfer in one kind of “real homodimer” with a pair of carboxyl groups. Measure the rotational transitions of those with “zero dipole moment”.

Calculated rotational constants and structure of formic acid dimer (DCOOH-HCOOH) H μa: 10 -4~10 -2 D D MP 2/6 -311++G** A: 6047. 6 MHz B: 2141. 9 MHz C: 1581. 7 MHz B 3 LYP/6 -311++G** A: 6069. 2 MHz B: 2168. 7 MHz C: 1597. 7 MHz M. Oldani, A. Bauder, Pure Rotational Spectrum of Benzene-d 1, CPC, 108, 1984

Fourier transform microwave spectrometer Covering range: 6. 5 ─18 GHz Resolution: 5 k. Hz Sensitivity: 10 -10 - 10 -12 cm-1 Operating Band: 6 -18 GHz Saturated Power: 0. 1 Watts Excitation Width: 1. 1 μs Pressure: 2 bar → ~10 -5 mbar Operating Band: 2 -20 GHz Saturated Power: 20 Watts Temperature: 1 K Excitation Width: 6 μs Accumulation of each spectrum: 10, 000 -30, 000 cycles W. Caminati, A. Millemaggi, J. L. Alonso, A. Lesarri, J. C. Lopez, S. Mata, Chem. Phys. Lett. 2004, 392, 1

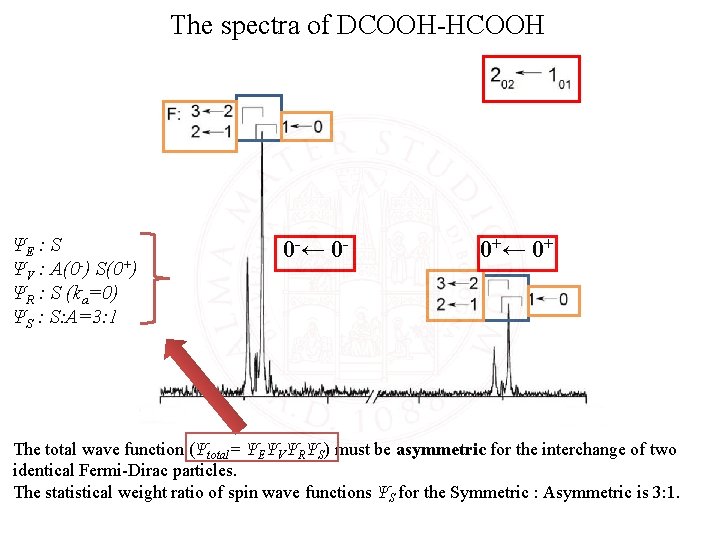
The spectra of DCOOH-HCOOH ΨE : S ΨV : A(0 -) S(0+) ΨR : S (ka=0) ΨS : S: A=3: 1 0 -← 0 - 0+← 0+ The total wave function (Ψtotal= ΨEΨVΨRΨS) must be asymmetric for the interchange of two identical Fermi-Dirac particles. The statistical weight ratio of spin wave functions ΨS for the Symmetric : Asymmetric is 3: 1.

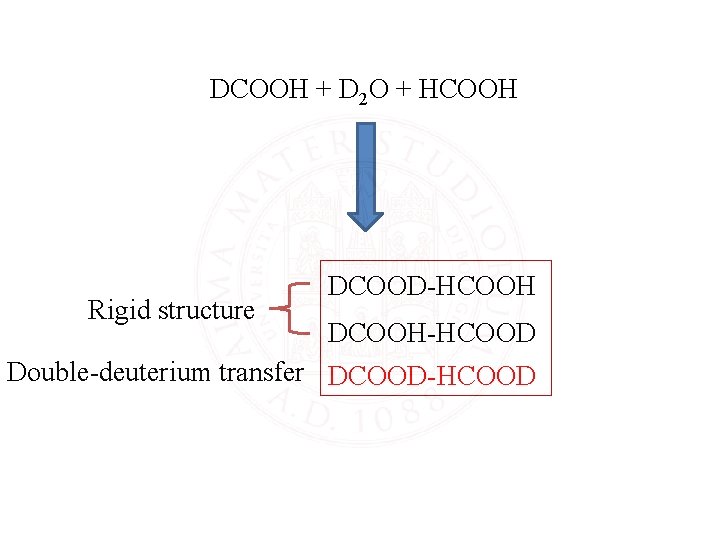
DCOOH + D 2 O + HCOOH Rigid structure DCOOD-HCOOH DCOOH-HCOOD Double-deuterium transfer DCOOD-HCOOD

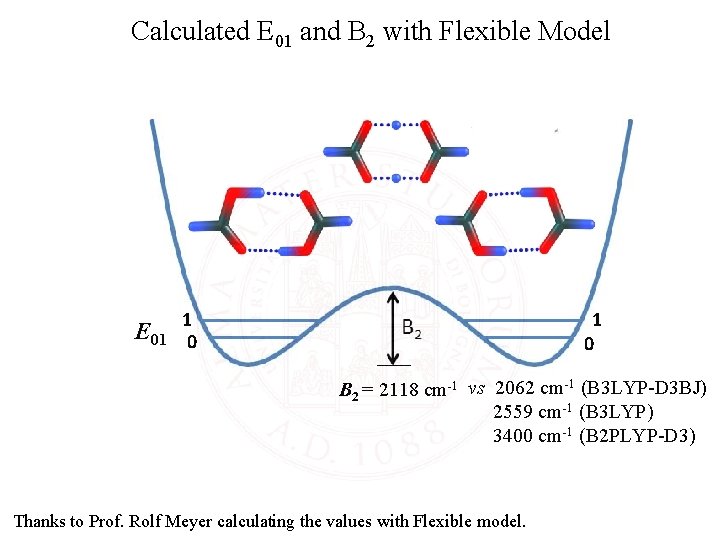
Calculated E 01 and B 2 with Flexible Model 1 1 0 E 01 0 B 2 = 2118 cm-1 vs 2062 cm-1 (B 3 LYP-D 3 BJ) 2559 cm-1 (B 3 LYP) 3400 cm-1 (B 2 PLYP-D 3) Thanks to Prof. Rolf Meyer calculating the values with Flexible model.

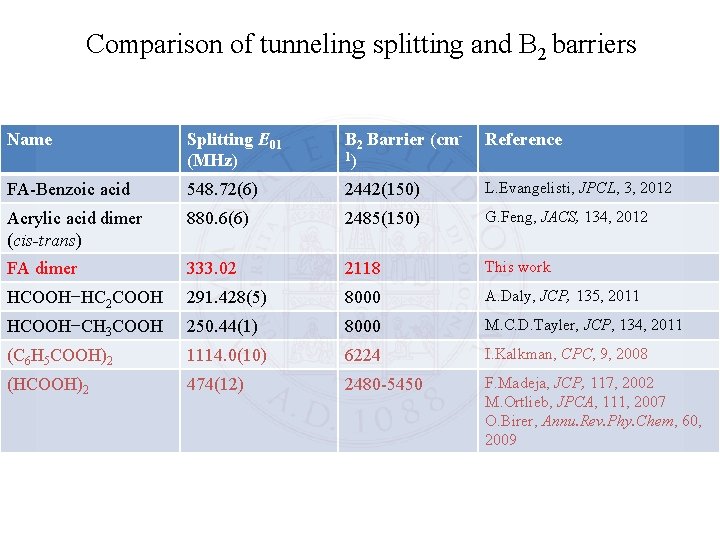
Comparison of tunneling splitting and B 2 barriers Name Splitting E 01 (MHz) B 2 Barrier (cm 1) Reference FA-Benzoic acid 548. 72(6) 2442(150) L. Evangelisti, JPCL, 3, 2012 Acrylic acid dimer (cis-trans) 880. 6(6) 2485(150) G. Feng, JACS, 134, 2012 FA dimer 333. 02 2118 This work HCOOH−HC 2 COOH 291. 428(5) 8000 A. Daly, JCP, 135, 2011 HCOOH−CH 3 COOH 250. 44(1) 8000 M. C. D. Tayler, JCP, 134, 2011 (C 6 H 5 COOH)2 1114. 0(10) 6224 I. Kalkman, CPC, 9, 2008 (HCOOH)2 474(12) 2480 -5450 F. Madeja, JCP, 117, 2002 M. Ortlieb, JPCA, 111, 2007 O. Birer, Annu. Rev. Phy. Chem, 60, 2009

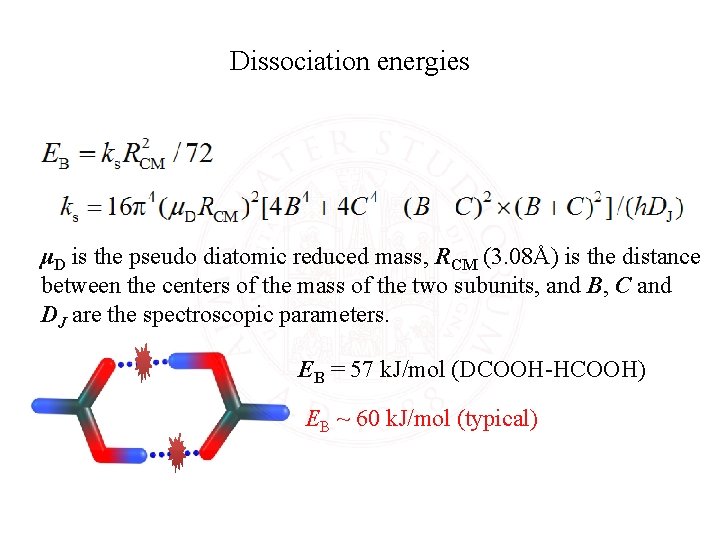
Dissociation energies μD is the pseudo diatomic reduced mass, RCM (3. 08Å) is the distance between the centers of the mass of the two subunits, and B, C and DJ are the spectroscopic parameters. EB = 57 k. J/mol (DCOOH-HCOOH) EB ~ 60 k. J/mol (typical)

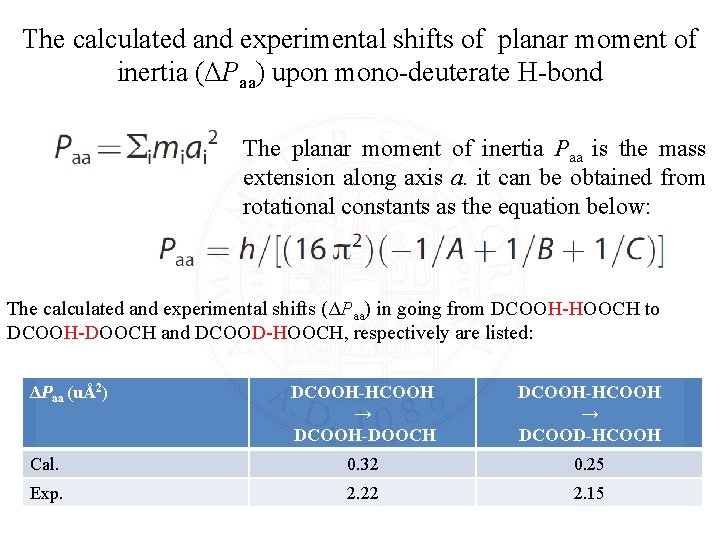
The calculated and experimental shifts of planar moment of inertia (ΔPaa) upon mono-deuterate H-bond The planar moment of inertia Paa is the mass extension along axis a. it can be obtained from rotational constants as the equation below: The calculated and experimental shifts (ΔPaa) in going from DCOOH-HOOCH to DCOOH-DOOCH and DCOOD-HOOCH, respectively are listed: ΔPaa (uÅ2) DCOOH-HCOOH → DCOOH-DOOCH DCOOH-HCOOH → DCOOD-HCOOH Cal. 0. 32 0. 25 Exp. 2. 22 2. 15

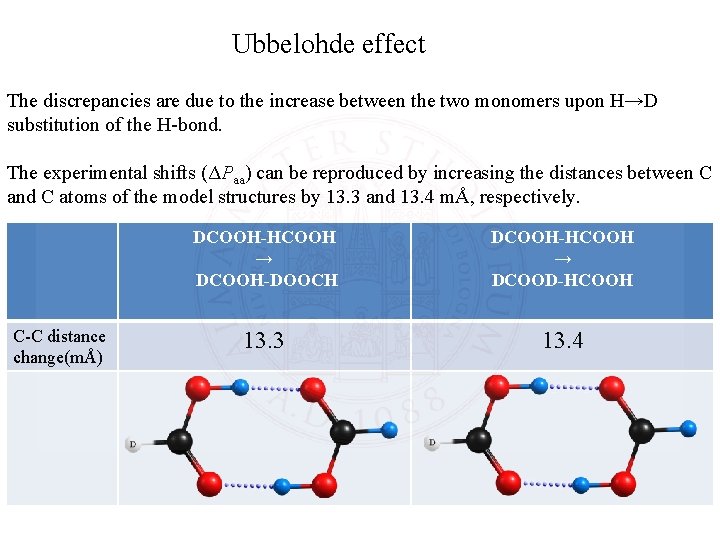
Ubbelohde effect The discrepancies are due to the increase between the two monomers upon H→D substitution of the H-bond. The experimental shifts (ΔPaa) can be reproduced by increasing the distances between C and C atoms of the model structures by 13. 3 and 13. 4 mÅ, respectively. C-C distance change(mÅ) DCOOH-HCOOH → DCOOH-DOOCH DCOOH-HCOOH → DCOOD-HCOOH 13. 3 13. 4

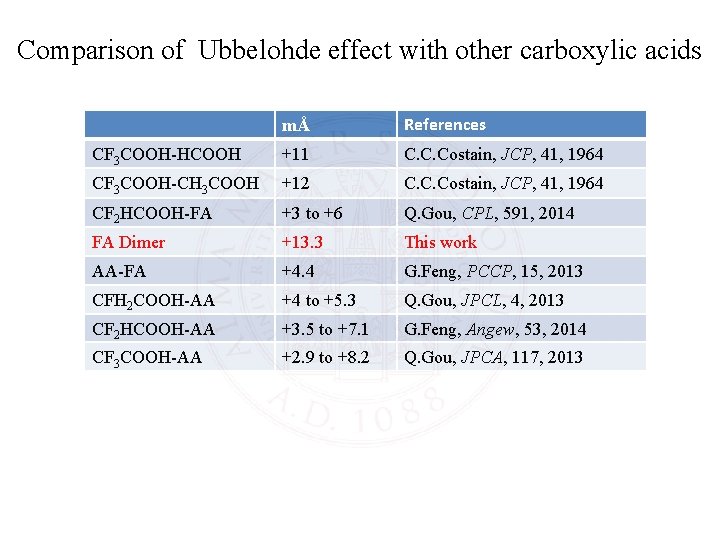
Comparison of Ubbelohde effect with other carboxylic acids mÅ References CF 3 COOH-HCOOH +11 C. C. Costain, JCP, 41, 1964 CF 3 COOH-CH 3 COOH +12 C. C. Costain, JCP, 41, 1964 CF 2 HCOOH-FA +3 to +6 Q. Gou, CPL, 591, 2014 FA Dimer +13. 3 This work AA-FA +4. 4 G. Feng, PCCP, 15, 2013 CFH 2 COOH-AA +4 to +5. 3 Q. Gou, JPCL, 4, 2013 CF 2 HCOOH-AA +3. 5 to +7. 1 G. Feng, Angew, 53, 2014 CF 3 COOH-AA +2. 9 to +8. 2 Q. Gou, JPCA, 117, 2013

To Conclude… The tunneling splitting due to the concerted double proton transfer in DCOOH-HCOOH has been measured with MW spectroscopy. The energy splitting was obtained as well as the B 2 barrier. The mono-deuterated species in H-bond were rotational studied.

Acknowledgements Prof. Walther Caminati Prof. Rolf Meyer Prof. Sonia Melandri Dr. Luca Evangelisti Dr. Qian Gou Dr. Assimo Maris Dr. Laura Favero Dr. Camilla Calabrese Dr. Annalisa Vigorito China Scholarship Council
 Rotational equilibrium and rotational dynamics
Rotational equilibrium and rotational dynamics Torque right hand rule
Torque right hand rule Dr dimer
Dr dimer D dimer test
D dimer test High d dimer
High d dimer D-dimer kantitatif
D-dimer kantitatif Primer dimer
Primer dimer Pyrimidine dimer
Pyrimidine dimer Modifiye wells skoru
Modifiye wells skoru Dimer san raffaele telefono
Dimer san raffaele telefono Cyclobutane thymine dimer
Cyclobutane thymine dimer Tromboplastin
Tromboplastin Orbital diagram for cu
Orbital diagram for cu Absortpion
Absortpion Pure spectrum of light
Pure spectrum of light Nonpolar hydrophobic
Nonpolar hydrophobic Nh3 shape and polarity
Nh3 shape and polarity Bond polarity definition
Bond polarity definition Polarity of chromatography paper
Polarity of chromatography paper