The Development of Atomic Theory SCH 12 U






- Slides: 22

The Development of Atomic Theory SCH 12 U September 7 2011 Mr. Dvorsky

• Nearly 2500 years ago Greek philosophers (i. e. Democritus) expressed a belief matter is composed of tiny indivisible particles called atoms (atomos is the Greek word for “indivisible”) • These conclusions were not based on any evidence; they were derived from philosophical reasoning.

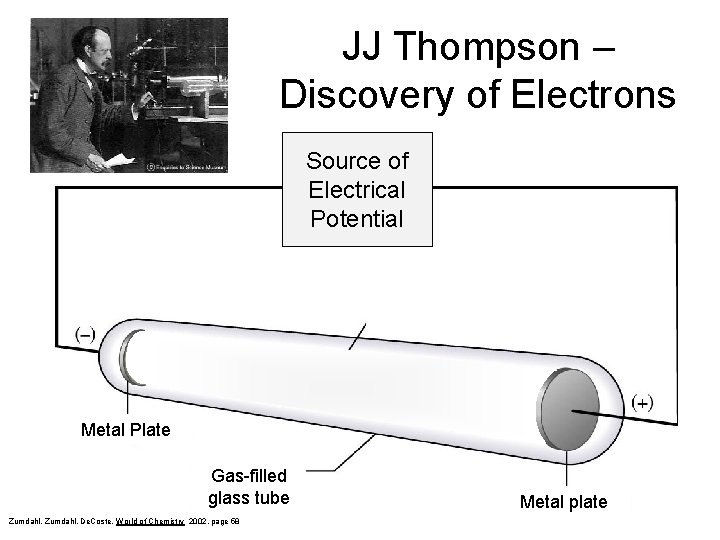
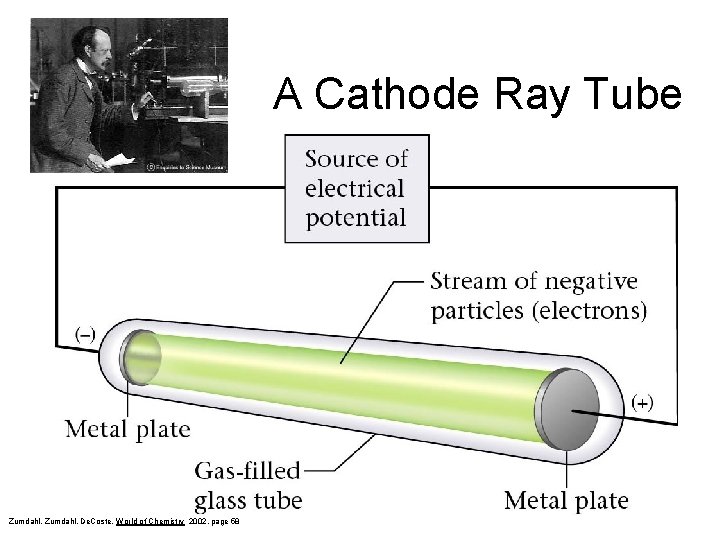
JJ Thompson – Discovery of Electrons Source of Electrical Potential Stream of negative particles (electrons) Metal Plate Gas-filled glass tube Zumdahl, De. Coste, World of Chemistry 2002, page 58 Metal plate

A Cathode Ray Tube Zumdahl, De. Coste, World of Chemistry 2002, page 58

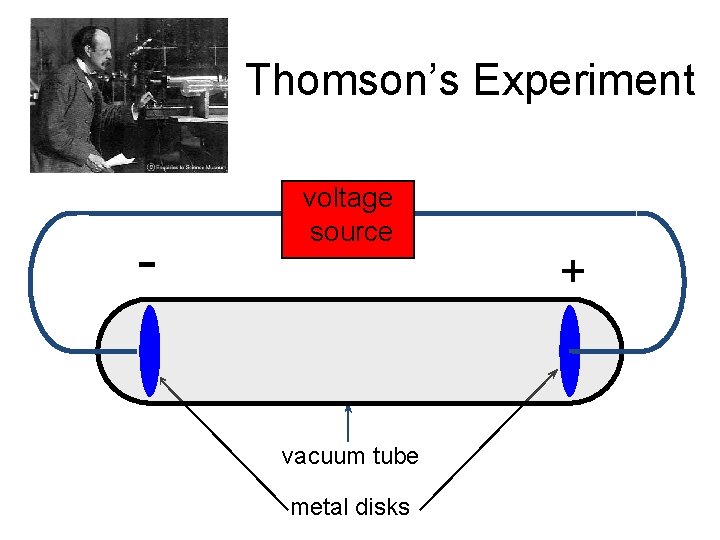
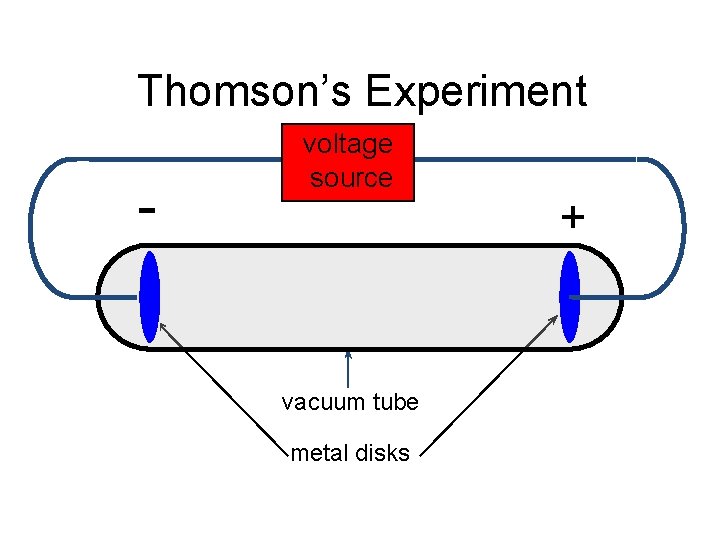
Thomson’s Experiment - voltage source vacuum tube metal disks +

Thomson’s Experiment - voltage source vacuum tube metal disks +

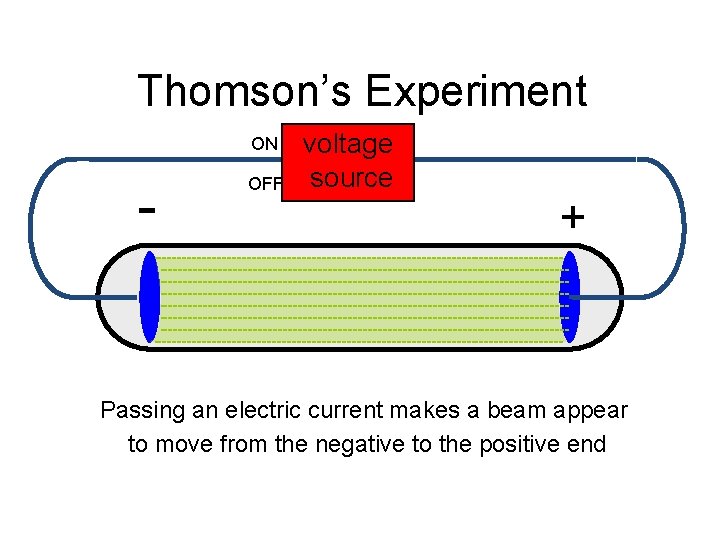
Thomson’s Experiment ON - OFF voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

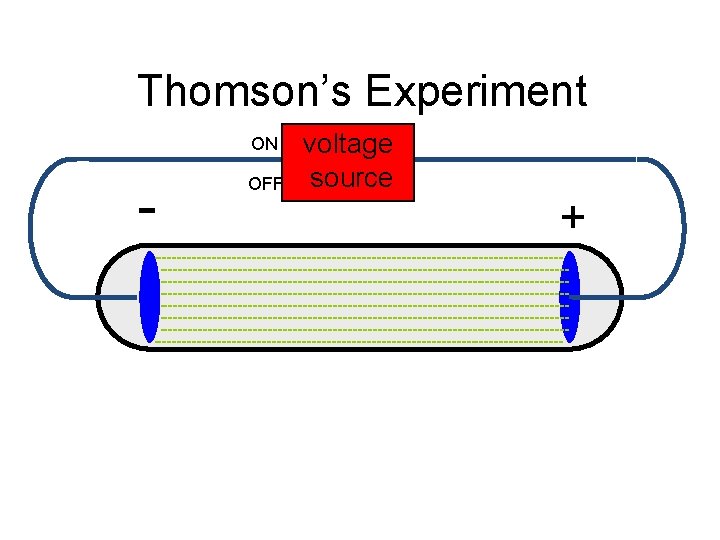
Thomson’s Experiment ON - OFF voltage source +

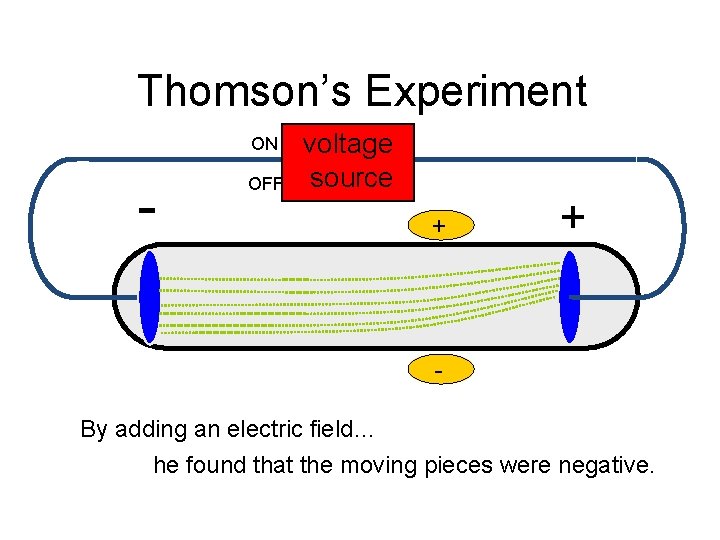
Thomson’s Experiment ON - OFF voltage source + + By adding an electric field… he found that the moving pieces were negative.

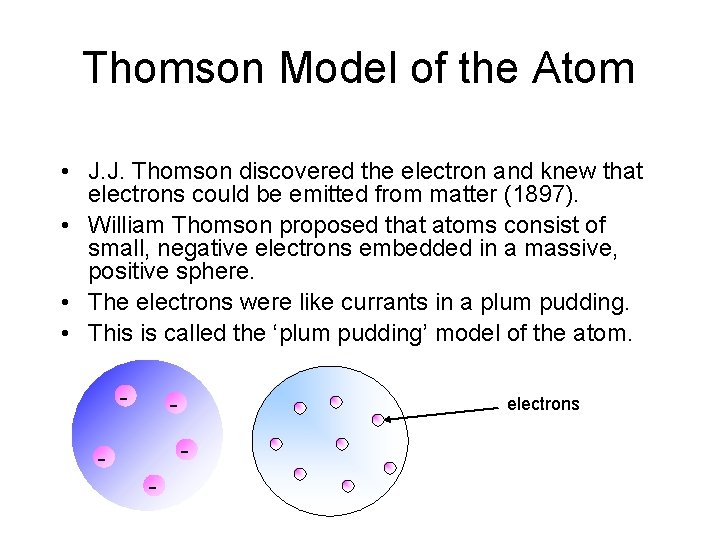
J. J. Thomson • He proved that atoms of any element can be made to emit tiny negative particles. • From this he concluded that ALL atoms must contain these negative particles. • He knew that atoms did not have a net negative charge and so there must be balancing the negative charge.

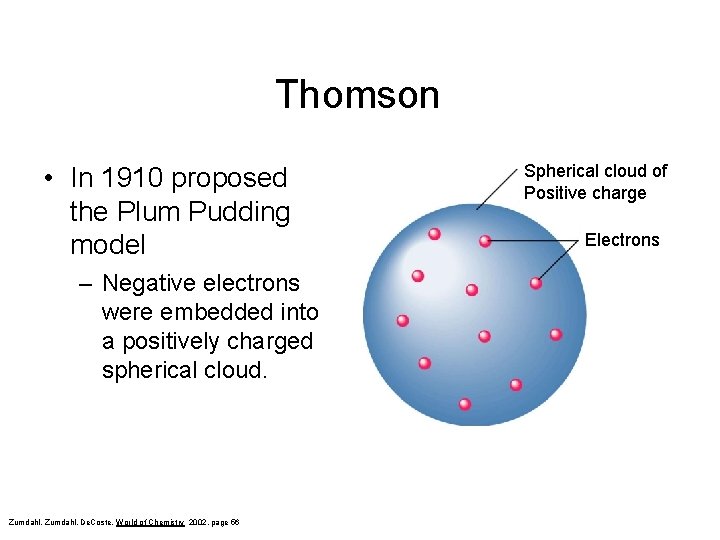
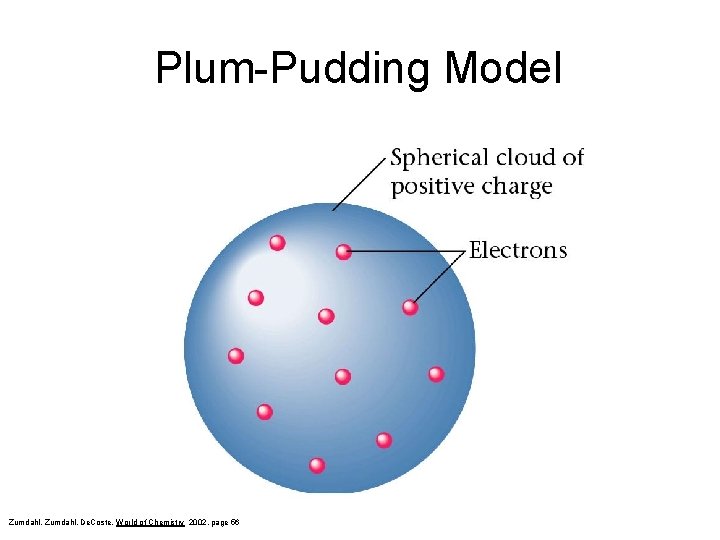
Thomson • In 1910 proposed the Plum Pudding model – Negative electrons were embedded into a positively charged spherical cloud. Zumdahl, De. Coste, World of Chemistry 2002, page 56 Spherical cloud of Positive charge Electrons

Plum-Pudding Model Zumdahl, De. Coste, World of Chemistry 2002, page 56

Thomson Model of the Atom • J. J. Thomson discovered the electron and knew that electrons could be emitted from matter (1897). • William Thomson proposed that atoms consist of small, negative electrons embedded in a massive, positive sphere. • The electrons were like currants in a plum pudding. • This is called the ‘plum pudding’ model of the atom. - - electrons - -

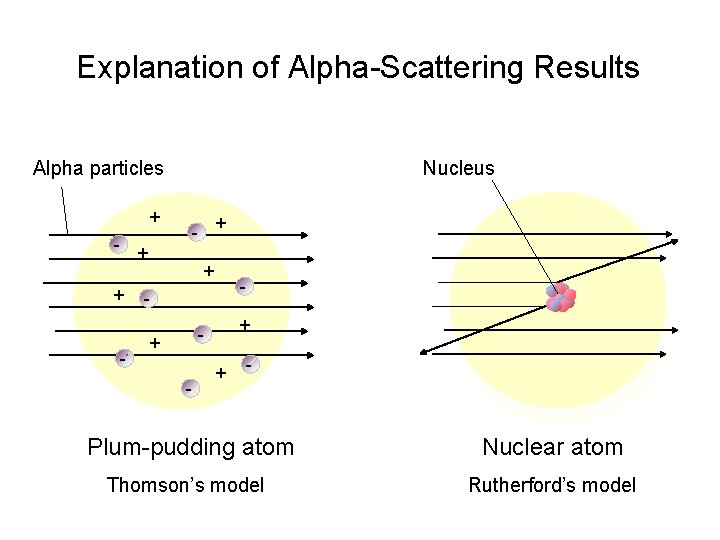
Rutherford, see animation -fired alpha particles at a very thin piece of foil. -The alpha particles to pass through without changing direction (very much) Because. . -The positive charges were spread out evenly. Alone they were not enough to stop the

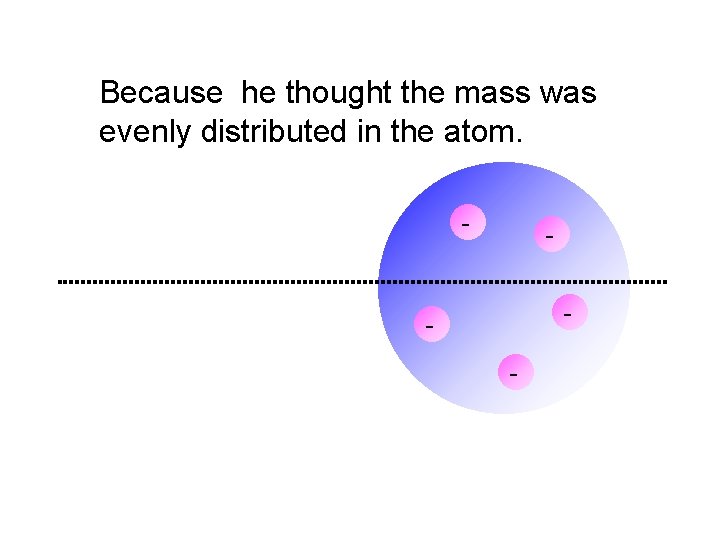
Because he thought the mass was evenly distributed in the atom. - - -

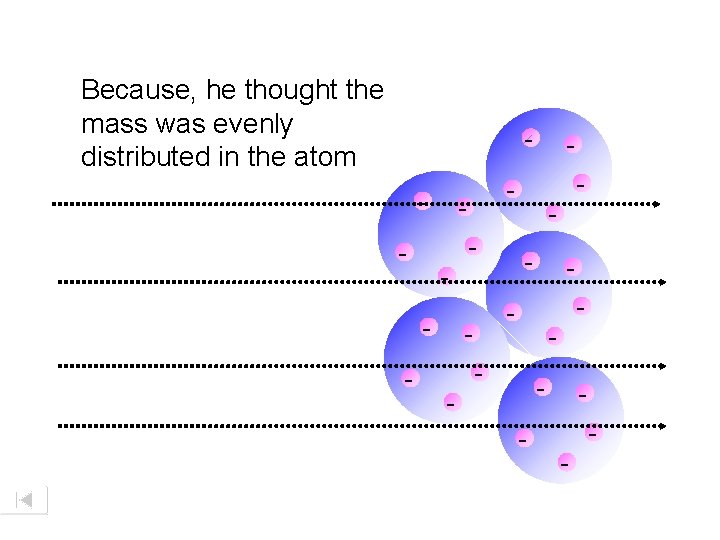
Because, he thought the mass was evenly distributed in the atom - - - - -

Explanation of Alpha-Scattering Results Alpha particles Nucleus + - + + - - + - + - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model

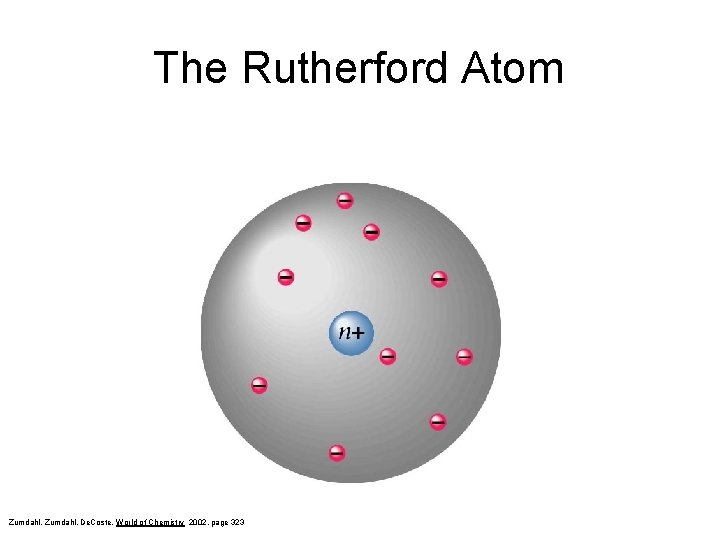
The Rutherford Atom Zumdahl, De. Coste, World of Chemistry 2002, page 323

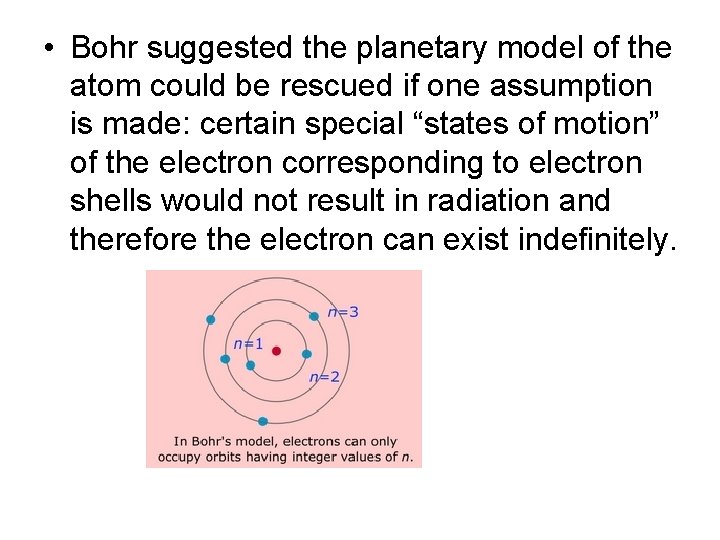
• Bohr suggested the planetary model of the atom could be rescued if one assumption is made: certain special “states of motion” of the electron corresponding to electron shells would not result in radiation and therefore the electron can exist indefinitely.

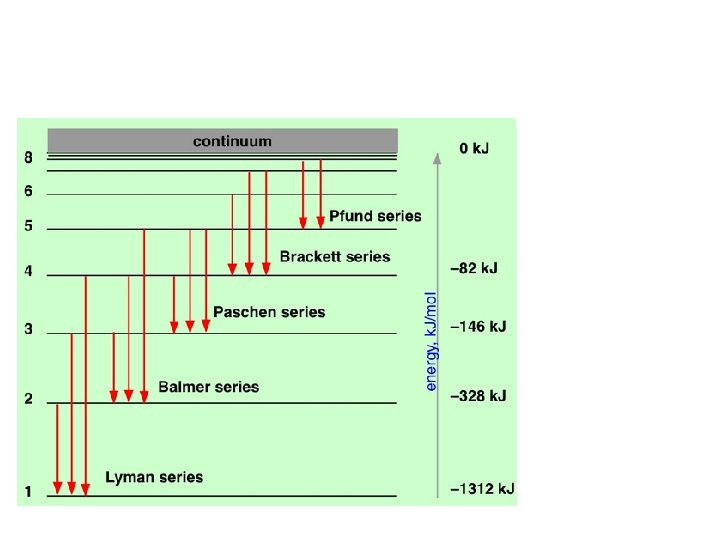
Bohr was saying, in effect, is that the atom can exist only in certain discrete energy states: the energy of the atom is quantized. Bohr noted that this quantization nicely explained the observed emission spectrum of the hydrogen atom. The electron is normally in its smallest allowed orbit, corresponding to n = 1; upon excitation in an electrical discharge or

These higher excited states of the atom are unstable, so after a very short time (around 10— 9 sec) the electron falls into lower orbits and finally into the innermost one, which corresponds to the atom's ground state. The energy lost on each jump is given off as a photon, and the frequency of this light provides a direct experimental measurement of the difference in the energies of the two states, according to the Planck-Einstein
