The CLEVER Trial Exercise versus Endovascular Revascularization Implications






























- Slides: 54

The CLEVER Trial: Exercise versus Endovascular Revascularization Implications for Management of PAD Diane Treat Jacobson, Ph. D, RN, FAHA, FSVM, FAAN, Professor University of Minnesota School of Nursing

Disclosures • None

Acknowledgements The CLEVER Study was sponsored by the National Heart Lung and Blood Institute (grants HL 77221 and HL 081656) Financial support was received from Cordis/Johnson & Johnson (Warren, NJ), e. V 3 (Plymouth, MN), and Boston Scientific (Natick, MA). Otsuka America, Inc. , (San Francisco, CA) donated cilostazol for all study participants throughout the study. Omron Healthcare Inc. , Lake Forest, IL donated pedometers. Krames Staywell, San Bruno, CA, donated print materials for study participants on exercise and diet.

CLEVER Study Investigators Timothy P. Murphy, MD; Donald E. Cutlip, MD; Judith G. Regensteiner, Ph. D; Emile R. Mohler, MD; David J. Cohen, MD; Matthew R. Reynolds, MD, MSc; Joseph M. Massaro, Ph. D; Beth A. Lewis, Ph. D; Joselyn Cerezo, MD; Niki C. Oldenburg, Dr. PH. ; Claudia C. Thum, MA; Suzanne Goldberg, MSN; Michael R. Jaff, DO; Michael W. Steffes, MD; Anthony J. Comerota, MD; Jonathan Ehrman, Ph. D; Diane Treat Jacobson, RN, Ph. D; M. Eileen Walsh, RN, Ph. D; Tracie Collins, MD; Dalynn T. Badenhop, Ph. D; Ulf Bronas, Ph. D; Alan T. Hirsch, MD

• CLaudication: Exercise Vs. Endoluminal Revascularization • Prospective multicenter randomized clinical trial that evaluated the relative efficacy and safety of stenting plus optimal medical therapy versus supervised exercise training plus optimal medical therapy versus optimal medical therapy alone in patients with aortoiliac disease.


Background ● Invasive stent procedures have not been shown to offer better claudication improvement than supervised exercise ● Reimbursement ● Stenting is reimbursed ● Supervised exercise is not ● Patients with proximal (aortoiliac) PAD ● highly symptomatic ● generally considered ideal for stent revascularization

CLEVER Objectives • To test whether aortoiliac stenting (ST) or supervised exercise therapy (SE) were superior to optimal medical care (OMC) as measured by peak walking time (PWT) at 6 months in patients with claudication due to aortoiliac peripheral artery disease (PAD). • If these comparisons were positive, to test whether stenting was superior to supervised exercise for the same endpoint.

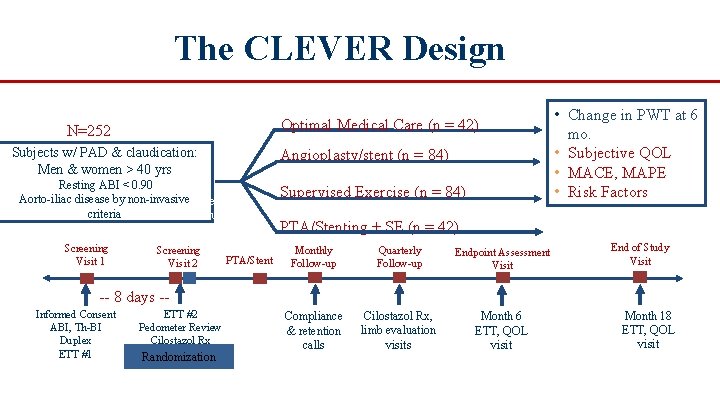
The CLEVER Design Optimal Medical Care (n = 42) N=252 Subjects w/ PAD & claudication: Men & women > 40 yrs Angioplasty/stent (n = 84) Resting ABI < 0. 90 Aorto iliac disease by non invasive. Screening criteria Evaluation Screening Visit 1 Screening Visit 2 PTA/Stent Supervised Exercise (n = 84) Supervised • Change in PWT at 6 mo. • Subjective QOL • MACE, MAPE • Risk Factors I Adherence I PTA/Stenting + SE (n = 42) Monthly Follow up Quarterly Follow up Endpoint Assessment Visit Compliance & retention calls Cilostazol Rx, limb evaluation visits Month 6 ETT, QOL visit End of Study Visit 8 days Informed Consent ABI, Th BI Duplex ETT #1 ETT #2 Pedometer Review Cilostazol Rx Randomization Month 18 ETT, QOL visit

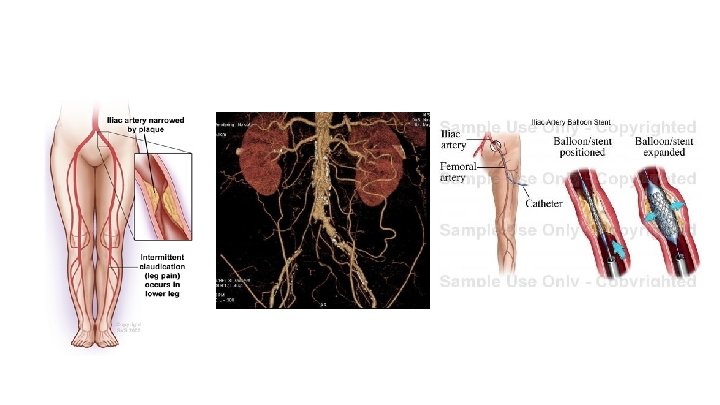
The CLEVER Population ● Moderate to severe claudication ● 2 -11 min on Gardner treadmill protocol or up to 5. 5 METS ● Hemodynamically significant aortoiliac PAD confirmed by non invasive vascular lab testing or advanced imaging ● No other co morbid diseases that limited walking ● No critical limb ischemia ● rest pain, non-healing wound or gangrene ● Superficial femoral artery disease was allowed and endovascular treatment permitted by protocol.

Treatment Strategies Optimal Medical Care (OMC): ● Cilostazol 100 mg b. i. d. as tolerated ● written and oral advice about exercise and diet ● monthly coordinator contact Supervised Exercise (SE): ● OMC plus 78 sessions of supervised exercise ● 3 x/wk, for 1 hr sessions Stenting (ST): ● OMC plus stent revascularization of aortoiliac PAD

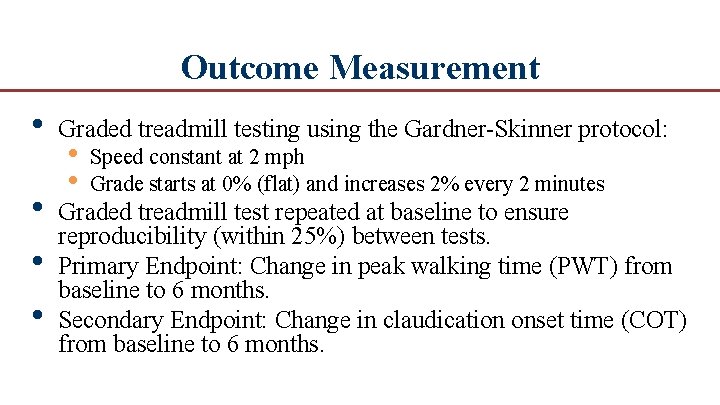
Outcome Measurement • Graded treadmill testing using the Gardner Skinner protocol: • • • Graded treadmill test repeated at baseline to ensure reproducibility (within 25%) between tests. Primary Endpoint: Change in peak walking time (PWT) from baseline to 6 months. Secondary Endpoint: Change in claudication onset time (COT) from baseline to 6 months. • • Speed constant at 2 mph Grade starts at 0% (flat) and increases 2% every 2 minutes

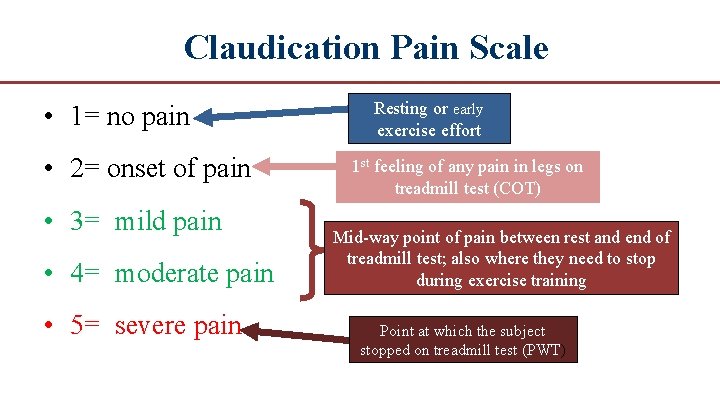
Claudication Pain Scale • 1= no pain • 2= onset of pain • 3= mild pain • 4= moderate pain • 5= severe pain Resting or early exercise effort 1 st feeling of any pain in legs on treadmill test (COT) Mid way point of pain between rest and end of treadmill test; also where they need to stop during exercise training Point at which the subject stopped on treadmill test (PWT)

Outcome Measurement • Additional Secondary Endpoints: • • Change in Community Based Step Activity • Assessed by pedometer measurements over 7 consecutive days • Supervised Exercise participants did not wear pedometers during supervised exercise sessions Change in symptoms, functional status, and quality of life • SF 12 – physical health and mental health subscales ( 0 100) • Walking Impairment Questionnaire (WIQ) • Distance, Speed, and Stairs subscales (0 100) • Peripheral Artery Questionnaire (PAQ) • Physical limitation, symptoms, QL, social function, treatment satisfaction, and summary score (0 100)

Supervised Exercise Regimen for PAD: Training Principles • Frequency • • 3 times a week supervised, included warm up; cool down & additional walking at home 2+ times per week Build up walking time in a 50 min period • 26 weeks (78 sessions) • Type of exercise • Treadmill walking • Duration

Supervised Exercise Regimen for PAD: Training Principles • • Training Intensity Initial • Set by baseline treadmill test: • • 2 mph and grade that brings on claudication Stop walking and sit when reach “ 3” or “ 4” on claudication scale Resume when pain is completely gone Repeat process Subsequent • Progressive increases in grade and speed over time according to CLEVER study progression rules

Supervised Exercise Regimen for PAD: How to Increase Exercise Work Load • If participant can walk at their current work load (treadmill grade and speed) for 8 minutes: • Increase grade (by 2% increments) up to 10% over time • After 10% grade is reached, increase speed by 0. 2 mph up to 3 mph over time • Then increase grade by 2% increments up to 15%. • Increase speed by 0. 2 mph as tolerated

Exercise Sub-Committee • In person site training by a member of the Exercise Sub Committee • • • Ensure intervention fidelity Provided a point person to each site for troubleshooting Electronic monitoring of exercise training sessions to permit continuous feedback

Results

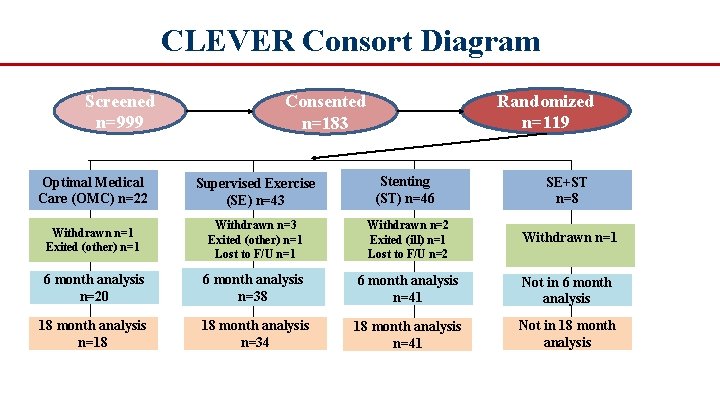
CLEVER Consort Diagram Screened n=999 Randomized n=119 Consented n=183 Optimal Medical Care (OMC) n=22 Supervised Exercise (SE) n=43 Stenting (ST) n=46 SE+ST n=8 Withdrawn n=1 Exited (other) n=1 Withdrawn n=3 Exited (other) n=1 Lost to F/U n=1 Withdrawn n=2 Exited (ill) n=1 Lost to F/U n=2 Withdrawn n=1 6 month analysis n=20 6 month analysis n=38 6 month analysis n=41 Not in 6 month analysis 18 month analysis n=18 18 month analysis n=34 18 month analysis n=41 Not in 18 month analysis

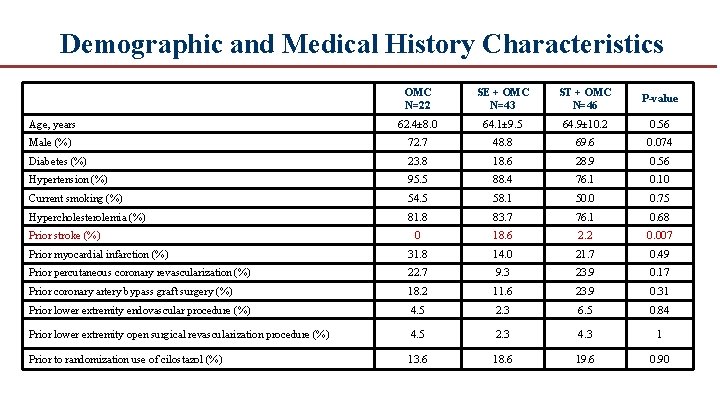
Demographic and Medical History Characteristics OMC N=22 SE + OMC N=43 ST + OMC N=46 P-value Age, years 62. 4± 8. 0 64. 1± 9. 5 64. 9± 10. 2 0. 56 Male (%) 72. 7 48. 8 69. 6 0. 074 Diabetes (%) 23. 8 18. 6 28. 9 0. 56 Hypertension (%) 95. 5 88. 4 76. 1 0. 10 Current smoking (%) 54. 5 58. 1 50. 0 0. 75 Hypercholesterolemia (%) 81. 8 83. 7 76. 1 0. 68 0 18. 6 2. 2 0. 007 Prior myocardial infarction (%) 31. 8 14. 0 21. 7 0. 49 Prior percutaneous coronary revascularization (%) 22. 7 9. 3 23. 9 0. 17 Prior coronary artery bypass graft surgery (%) 18. 2 11. 6 23. 9 0. 31 Prior lower extremity endovascular procedure (%) 4. 5 2. 3 6. 5 0. 84 Prior lower extremity open surgical revascularization procedure (%) 4. 5 2. 3 4. 3 1 Prior to randomization use of cilostazol (%) 13. 6 18. 6 19. 6 0. 90 Prior stroke (%)

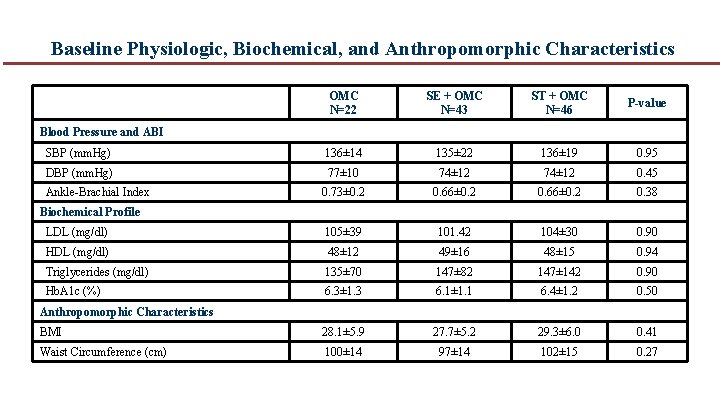
Baseline Physiologic, Biochemical, and Anthropomorphic Characteristics OMC N=22 SE + OMC N=43 ST + OMC N=46 P-value SBP (mm. Hg) 136± 14 135± 22 136± 19 0. 95 DBP (mm. Hg) 77± 10 74± 12 0. 45 0. 73± 0. 2 0. 66± 0. 2 0. 38 LDL (mg/dl) 105± 39 101. 42 104± 30 0. 90 HDL (mg/dl) 48± 12 49± 16 48± 15 0. 94 Triglycerides (mg/dl) 135± 70 147± 82 147± 142 0. 90 Hb. A 1 c (%) 6. 3± 1. 3 6. 1± 1. 1 6. 4± 1. 2 0. 50 BMI 28. 1± 5. 9 27. 7± 5. 2 29. 3± 6. 0 0. 41 Waist Circumference (cm) 100± 14 97± 14 102± 15 0. 27 Blood Pressure and ABI Ankle Brachial Index Biochemical Profile Anthropomorphic Characteristics

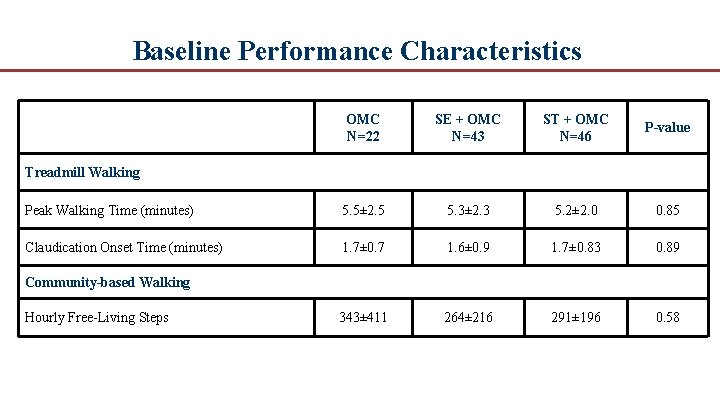
Baseline Performance Characteristics OMC N=22 SE + OMC N=43 ST + OMC N=46 P-value Peak Walking Time (minutes) 5. 5± 2. 5 5. 3± 2. 3 5. 2± 2. 0 0. 85 Claudication Onset Time (minutes) 1. 7± 0. 7 1. 6± 0. 9 1. 7± 0. 83 0. 89 343± 411 264± 216 291± 196 0. 58 Treadmill Walking Community-based Walking Hourly Free Living Steps

Treatment Delivery Cilostazol Compliance: • >90% in all treatment groups Exercise Compliance: • 29 (71%) of SE participants attended at least 70% of their 78 scheduled exercise sessions Technical Success of Stenting: • • • All ST patients were successfully stented Pre procedure mean lesion length 3. 9± 3. 4 cm Mean stenosis 83± 19%; post procedure stenosis 5± 8% ABI 0. 66+0. 2 at baseline, improved by 0. 29± 0. 33 No SFA Stents were performed on any study participant Crossover Rates: • None at six months

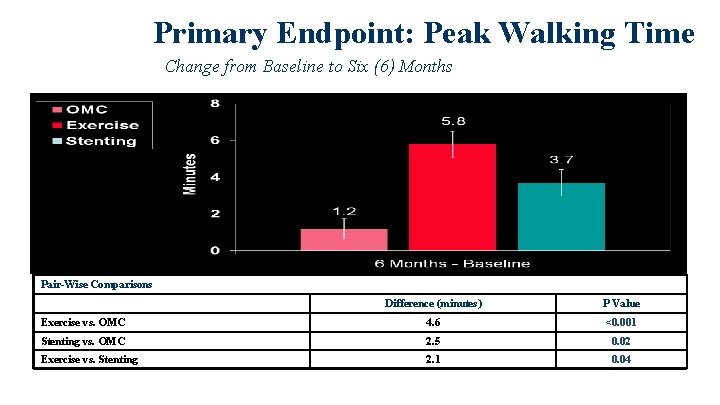
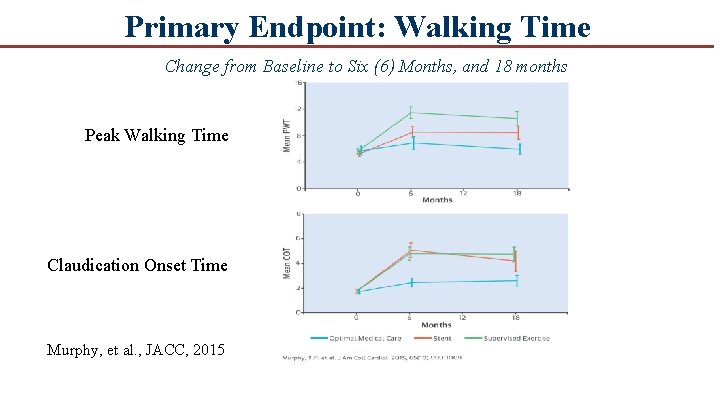
Primary Endpoint: Peak Walking Time Change from Baseline to Six (6) Months Pair-Wise Comparisons Difference (minutes) P Value Exercise vs. OMC 4. 6 <0. 001 Stenting vs. OMC 2. 5 0. 02 Exercise vs. Stenting 2. 1 0. 04

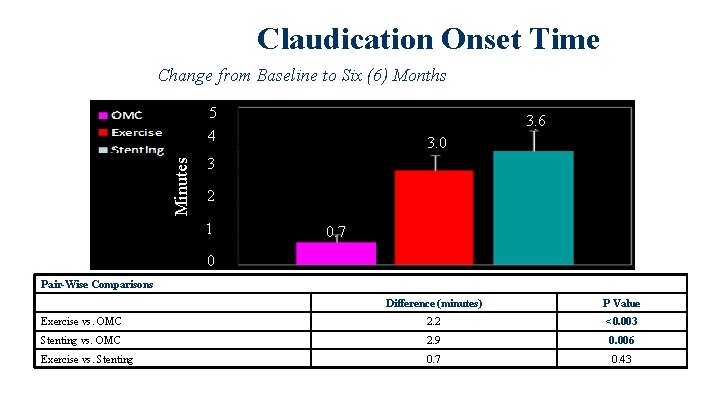
Claudication Onset Time Change from Baseline to Six (6) Months Minutes 5 4 3. 6 3. 0 3 2 1 0. 7 0 Pair-Wise Comparisons Difference (minutes) P Value Exercise vs. OMC 2. 2 <0. 003 Stenting vs. OMC 2. 9 0. 006 Exercise vs. Stenting 0. 7 0. 43

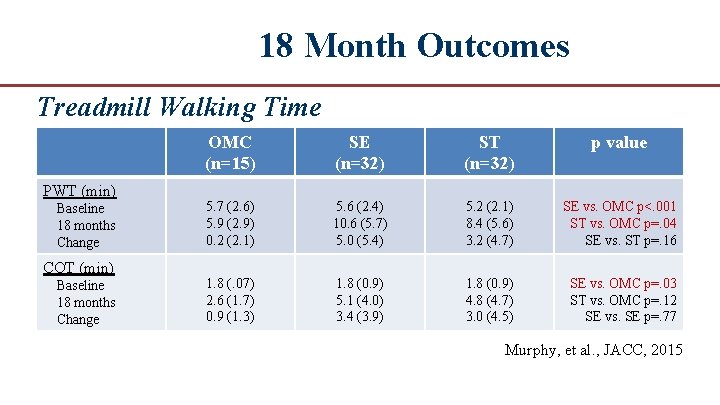
18 Month Outcomes Treadmill Walking Time PWT (min) Baseline 18 months Change COT (min) Baseline 18 months Change OMC (n=15) SE (n=32) ST (n=32) p value 5. 7 (2. 6) 5. 9 (2. 9) 0. 2 (2. 1) 5. 6 (2. 4) 10. 6 (5. 7) 5. 0 (5. 4) 5. 2 (2. 1) 8. 4 (5. 6) 3. 2 (4. 7) SE vs. OMC p<. 001 ST vs. OMC p=. 04 SE vs. ST p=. 16 1. 8 (. 07) 2. 6 (1. 7) 0. 9 (1. 3) 1. 8 (0. 9) 5. 1 (4. 0) 3. 4 (3. 9) 1. 8 (0. 9) 4. 8 (4. 7) 3. 0 (4. 5) SE vs. OMC p=. 03 ST vs. OMC p=. 12 SE vs. SE p=. 77 Murphy, et al. , JACC, 2015

Primary Endpoint: Walking Time Change from Baseline to Six (6) Months, and 18 months Peak Walking Time Claudication Onset Time Murphy, et al. , JACC, 2015

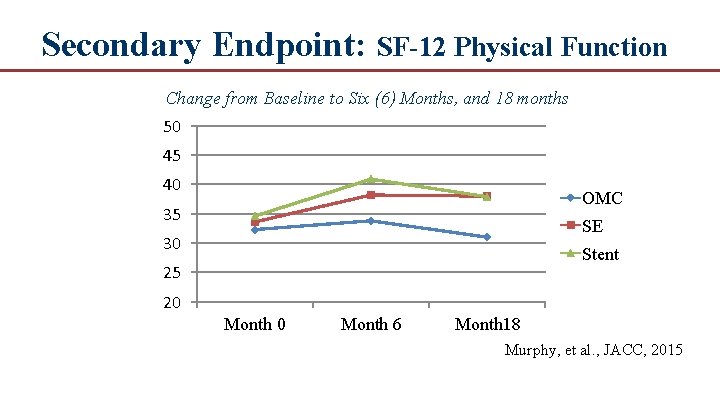
Secondary Endpoint: SF-12 Physical Function Change from Baseline to Six (6) Months, and 18 months 50 45 40 OMC 35 SE 30 Stent 25 20 Month 6 Month 18 Murphy, et al. , JACC, 2015

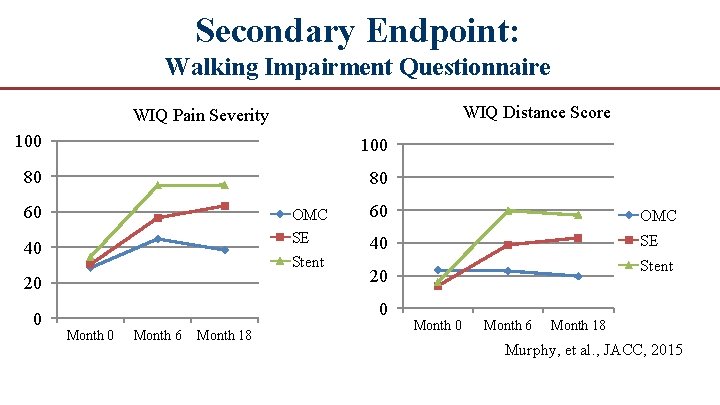
Secondary Endpoint: Walking Impairment Questionnaire WIQ Distance Score WIQ Pain Severity 100 80 80 60 OMC SE Stent 40 20 0 60 OMC 40 SE 0 Month 6 Month 18 Stent 20 Month 6 Month 18 Murphy, et al. , JACC, 2015

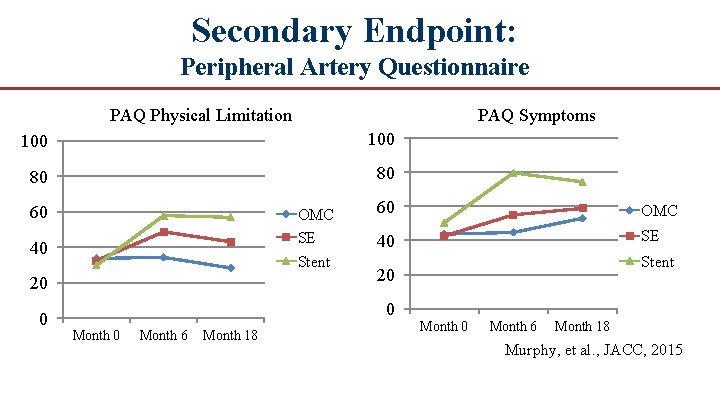
Secondary Endpoint: Peripheral Artery Questionnaire PAQ Physical Limitation PAQ Symptoms 100 80 80 60 OMC SE Stent 40 20 0 60 OMC 40 SE 20 0 Month 6 Month 18 Stent Month 0 Month 6 Month 18 Murphy, et al. , JACC, 2015

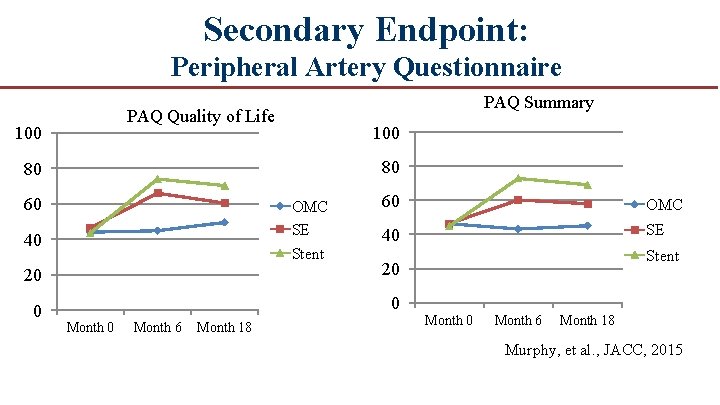
Secondary Endpoint: Peripheral Artery Questionnaire PAQ Summary PAQ Quality of Life 100 80 80 60 OMC SE Stent 40 60 OMC 40 SE 20 20 0 0 Month 6 Month 18 Stent Month 0 Month 6 Month 18 Murphy, et al. , JACC, 2015

Cost-Effectiveness • Pre planned analysis of cost effectiveness of supervised exercise (SE), stenting and optimal medical care (OMC) for claudication • Incremental cost effectiveness ratios (ICERS) • $24, 070 per quality adjusted life year gained for SE vs OMC • $41, 376 per quality adjusted life year gained for Stent vs OMC • $122, 600 per quality adjusted life year gained for Stent vs SE Reynolds, et al. , JAHA, 2014; 3: e 001233

Cost-Effectiveness “Given the increased expense and marginal benefits of ST relative to SE, there would appear to be no rational justification for covering ST but not SE for the treatment of claudication. ” (Reynolds, et al, p 8) Reynolds, et al. , JAHA, 2014; 3: e 001233

Conclusions ● In patients with moderate to severe claudication and hemodynamically significant aortoiliac disease, supervised exercise offers better treadmill walking performance outcomes than stent revascularization. ● Both supervised exercise and stenting are more effective at increasing walking distance compared to pharmacotherapy alone. ● Aortoiliac stent revascularization was associated with better QOL scores than patients treated with supervised exercise, which is unexplained. ● 18 month follow up data demonstrated that the treatment effects for both SE and ST groups were durable and superior to OMC and there was little difference between groups in walking outcomes, although some aspects of QOL remained higher in the ST group. ● Cost effectiveness analysis concluded that ST offers no significant benefit over SE and if ST is reimbursed by CMS SE should also be reimbursed

PAD The Real World Application Courtesy of Teresa Fitek, BA, CEP Fairview Ridges Hospital, Burnsville, MN

Fairview’s WEL Program • Peripheral Artery Disease Program is part of the “Wellness and Exercise for Life Program” • Self pay program (Scholarships are available) • CPT code for PAD Rehabilitation: 93668 • 1 hour initial evaluation includes: 6 min walk, brief medical history, and exercise. • Monitor: Rhythm strip, HR, BP • Stress tests frequently not available

Setting the initial workloads Stress test NOT available: • 6 min walk • Interview questions • Comfortable walking speed • Combination of all the above Stress test available: • 6 min walk for pre/post comparison • Initial speed is 2. 0 mph • % grade: Onset of claudication • MD may also establish the initial work levels • MET levels

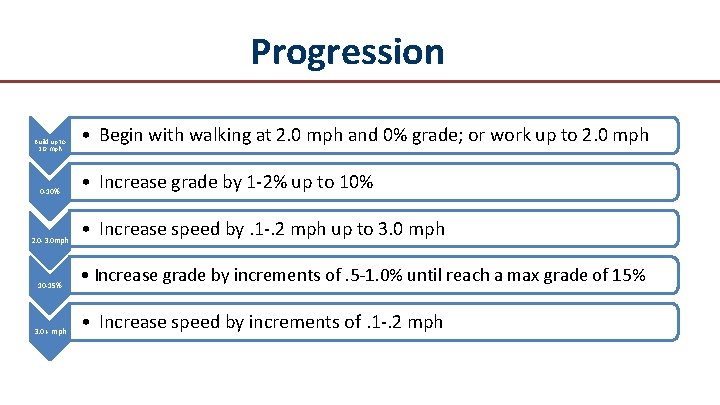
Progression Progress to next level when patient is able to walk a full 8 minutes without achieving moderate claudication pain

Progression Build up to 2. 0 mph 0 -10% 2. 0 -3. 0 mph 10 -15% 3. 0 + mph • Begin with walking at 2. 0 mph and 0% grade; or work up to 2. 0 mph • Increase grade by 1 -2% up to 10% • Increase speed by. 1 -. 2 mph up to 3. 0 mph • Increase grade by increments of. 5 -1. 0% until reach a max grade of 15% • Increase speed by increments of. 1 -. 2 mph

Monitoring • Tele-monitoring the initial session • Resting HR, BP recorded a minimum of once per week • Tele-monitor available if symptoms

Documentation • Initial evaluation includes a Walking Impairment Questionnaire (WIQ) and the Peripheral Artery Disease Quality of Life Survey Copyright: Diane Treat-Jacobson treat 001@umn. edu • Patients document their own treatment sessions • HR, BP minimum of one time per week • Workload, onset of pain, total walking time and rest periods

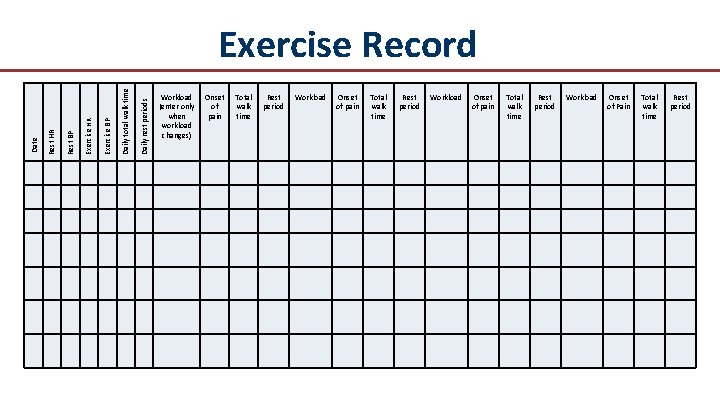
Daily rest periods Daily total walk time Exercise BP Exercise HR Rest BP Rest HR Date Exercise Record Workload (enter only when workload changes) Onset of pain Total walk time Rest period Workload Onset of Pain Total walk time Rest period

Home exercise program Patient encouraged to walk 4 -6 days per week including the days they attend the WEL program. • Treadmill • Mall walking • Outdoor walking Goal to walk at home until they reach moderate pain. Rest then walk again. Minimum of 30 min walking time

Benefits of Supervised Exercise • Safety • Confidence • Support

Case Study • 63 yr old female, DX: PAD • PT able to walk less than one block before developing claudication pain • Initial evaluation: PT reports being depressed. She no longer has the ability to clean her home, go on outings with her husband or play with her grandchildren. • Patient consented to a 3 month trial of the PAD WEL program

Case study • Patient tolerated 828 feet on the 6 min walk test. That is 317 feet less than predicted for her age • 6 min walk test determined patient was at 2. 2 METs. Initial treadmill level was set at 1. 6 mph (2. 2 METS) • Onset of pain was at 2 min. Due to time constraints on her initial session she was able to complete two walking bouts: 5: 26 and 4: 06 • Patient was encouraged to walk over the weekend. She was advised to walk per her tolerance up to 30 min of walking time. She was advised to stop walking and rest when she was at moderate claudication pain. Resume walking when claudication was back to baseline

Case Study • After the first full week of rehab, she excitedly reported she walked with her husband at the basketball game. She still had pain however it did not get as severe AND she was not worried about it. She told therapist that she has been advised to walk in the past. However, she was fearful of hurting herself further when she suffered significant pain in her legs. Thus she resorted to a sedentary lifestyle.

Case Study • The patient reached 8 min X 4 bouts on her 4 th session however she was not confident in herself at this point and did not want to increase to the next level. • Patient increased her exercise to 1. 8 mph on the 7 th session. She was still able to achieve the 8 min bouts and therefore therapist increased to 2. 0 mph the following session. • At 2. 0 mph patient was able to achieve 5 -6 minutes of walking time. However reported pain in her buttocks the next day. She was then brought back down to 1. 8 -1. 9 mph. As she was not comfortable at the 2. 0 mph.

Case Study • PT was in the WEL program for 3 months. • She improved her 6 min walk test by 38% and reports that she has her life back. She returned to grocery shopping, walking at social functions with her husband, and cleaning her own home. She reports that she still has claudication at higher levels of activity but is no longer afraid. She joined a Fitness center a few blocks from her house and continues to walk 5 days per week.

The Real World Application Depression Pain “walking hurts” Support, support Risk Factors: Smoking, Diabetes, High Cholesterol, Hypertension, sedentary lifestyle, age • Greatest risk factors for development of PAD is smoking and Diabetes. • •

Handouts Available • • Common Terms and Conditions Therapist Guidelines Exercise Record Home program guidelines

Future Studies • Study effects of real world PAD exercise therapy • Collect pre and post 6 minute walk data • Pain free walking time

Contact Information Diane Treat-Jacobson, Ph. D, RN, FAAN treat 001@umn. edu Teresa Fietek BA, CEP tfietek 1@fairview. org