AVERT Trial Atorvastatin Versus Revascularization Treatments AVERT Trial




- Slides: 17

AVERT Trial Atorvastatin Versus Revascularization Treatments (AVERT) Trial Presented at The American Heart Association Scientific Sessions 1998 Presented by Dr. Bertram Pitt

AVERT Trial: Background • The goal of the AVERT trial was to assess the effect of aggressive lipid-lowering therapy on ischemic events in low-risk patients with single- or doublevessel CAD. Presented at AHA 1998

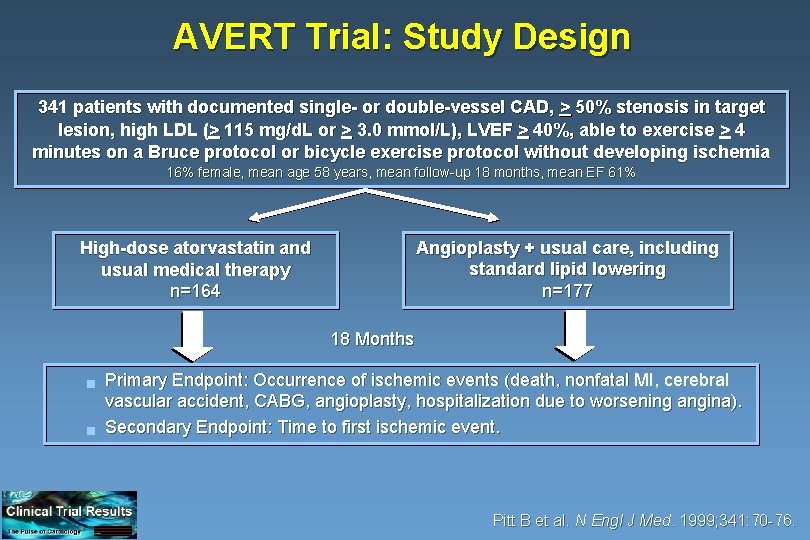
AVERT Trial: Study Design 341 patients with documented single- or double-vessel CAD, > 50% stenosis in target lesion, high LDL (> 115 mg/d. L or > 3. 0 mmol/L), LVEF > 40%, able to exercise > 4 minutes on a Bruce protocol or bicycle exercise protocol without developing ischemia 16% female, mean age 58 years, mean follow-up 18 months, mean EF 61% Angioplasty + usual care, including standard lipid lowering n=177 High-dose atorvastatin and usual medical therapy n=164 18 Months g g Primary Endpoint: Occurrence of ischemic events (death, nonfatal MI, cerebral vascular accident, CABG, angioplasty, hospitalization due to worsening angina). Secondary Endpoint: Time to first ischemic event. Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

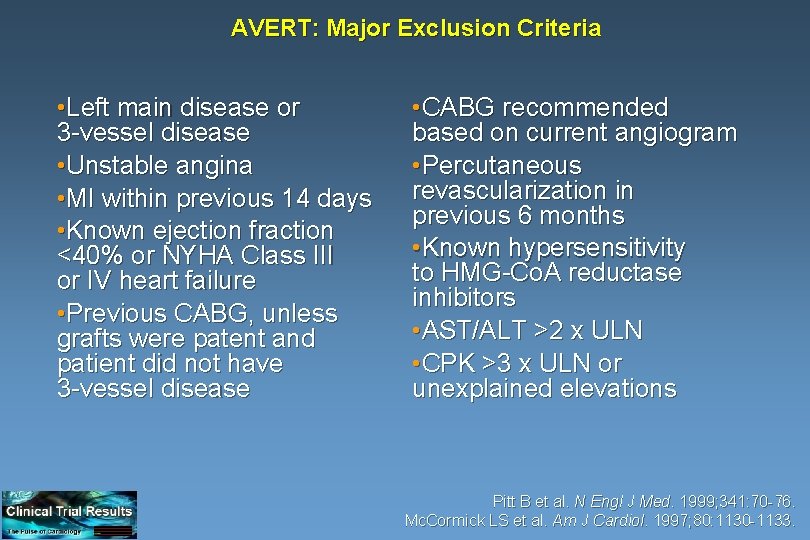
AVERT: Major Exclusion Criteria • Left main disease or 3 -vessel disease • Unstable angina • MI within previous 14 days • Known ejection fraction <40% or NYHA Class III or IV heart failure • Previous CABG, unless grafts were patent and patient did not have 3 -vessel disease • CABG recommended based on current angiogram • Percutaneous revascularization in previous 6 months • Known hypersensitivity to HMG-Co. A reductase inhibitors • AST/ALT >2 x ULN • CPK >3 x ULN or unexplained elevations Pitt B et al. N Engl J Med. 1999; 341: 70 -76. Mc. Cormick LS et al. Am J Cardiol. 1997; 80: 1130 -1133.

AVERT: Overview of Study Procedures Treatment phase • Patients randomized to atorvastatin – discontinued other lipid-lowering medication and immediately began atorvastatin 80 mg/d • Patients randomized to angioplasty/usual care (UC) – underwent angioplasty followed by “usual care” • usual care may or may not have included lipid-lowering therapy (eg, diet, behavior modification, or medication) • angioplasty may or may not have included stenting • usual care was determined by investigator or patient’s primary physician Pitt B et al. N Engl J Med. 1999; 341: 70 -76. Mc. Cormick LS et al. Am J Cardiol. 1997; 80: 1130 -1133.

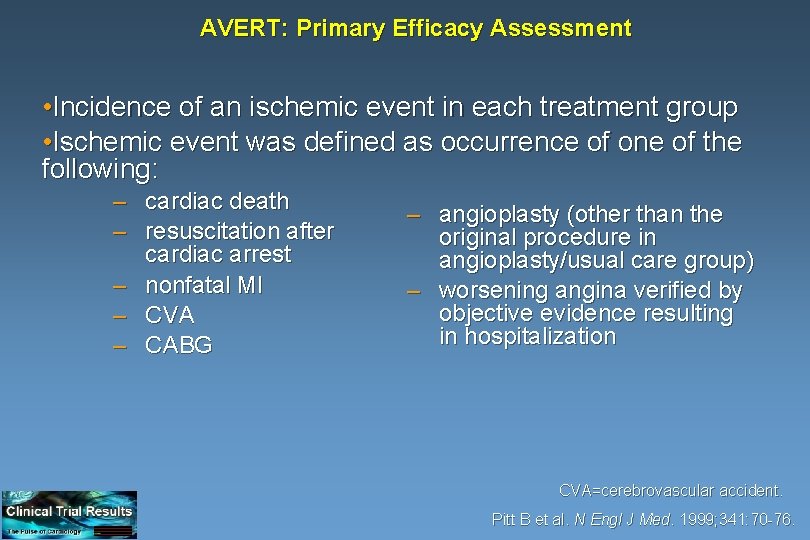
AVERT: Primary Efficacy Assessment • Incidence of an ischemic event in each treatment group • Ischemic event was defined as occurrence of one of the following: – cardiac death – resuscitation after cardiac arrest – nonfatal MI – CVA – CABG – angioplasty (other than the original procedure in angioplasty/usual care group) – worsening angina verified by objective evidence resulting in hospitalization CVA=cerebrovascular accident. Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

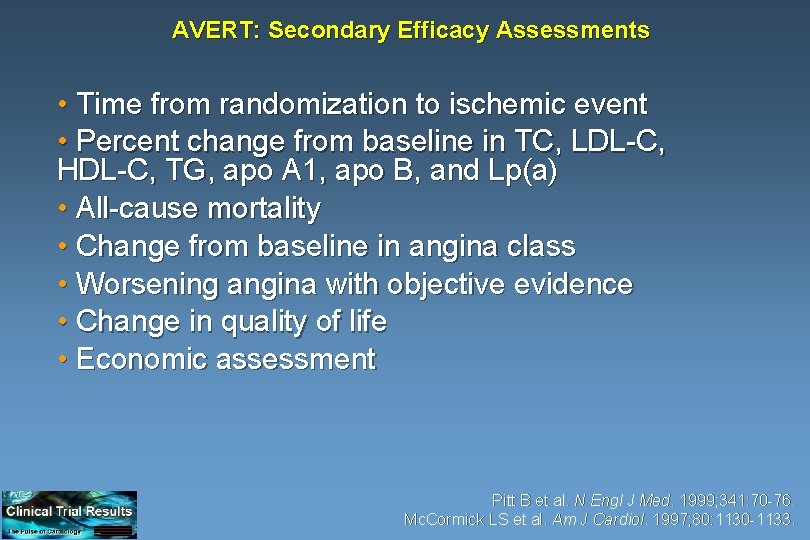
AVERT: Secondary Efficacy Assessments • Time from randomization to ischemic event • Percent change from baseline in TC, LDL-C, HDL-C, TG, apo A 1, apo B, and Lp(a) • All-cause mortality • Change from baseline in angina class • Worsening angina with objective evidence • Change in quality of life • Economic assessment Pitt B et al. N Engl J Med. 1999; 341: 70 -76. Mc. Cormick LS et al. Am J Cardiol. 1997; 80: 1130 -1133.

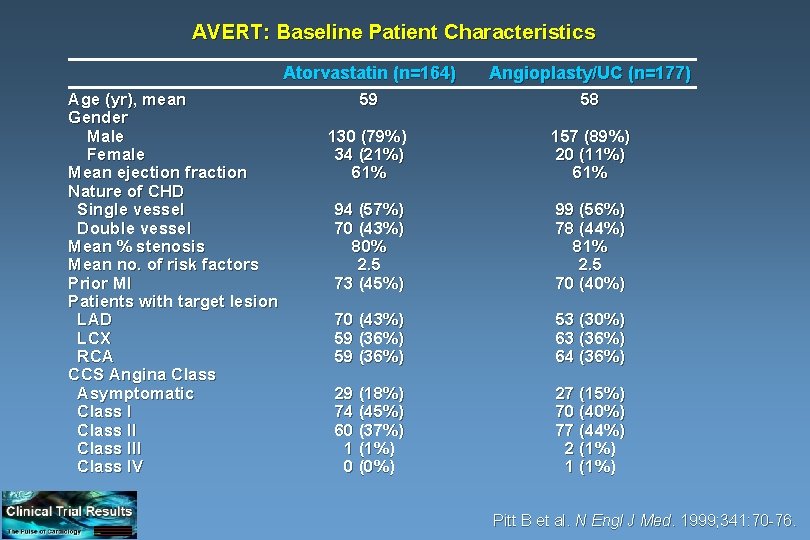
AVERT: Baseline Patient Characteristics Age (yr), mean Gender Male Female Mean ejection fraction Nature of CHD Single vessel Double vessel Mean % stenosis Mean no. of risk factors Prior MI Patients with target lesion LAD LCX RCA CCS Angina Class Asymptomatic Class III Class IV Atorvastatin (n=164) Angioplasty/UC (n=177) 59 58 130 (79%) 34 (21%) 61% 157 (89%) 20 (11%) 61% 94 (57%) 70 (43%) 80% 2. 5 73 (45%) 99 (56%) 78 (44%) 81% 2. 5 70 (40%) 70 (43%) 59 (36%) 53 (30%) 63 (36%) 64 (36%) 29 (18%) 74 (45%) 60 (37%) 1 (1%) 0 (0%) 27 (15%) 70 (40%) 77 (44%) 2 (1%) 1 (1%) Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

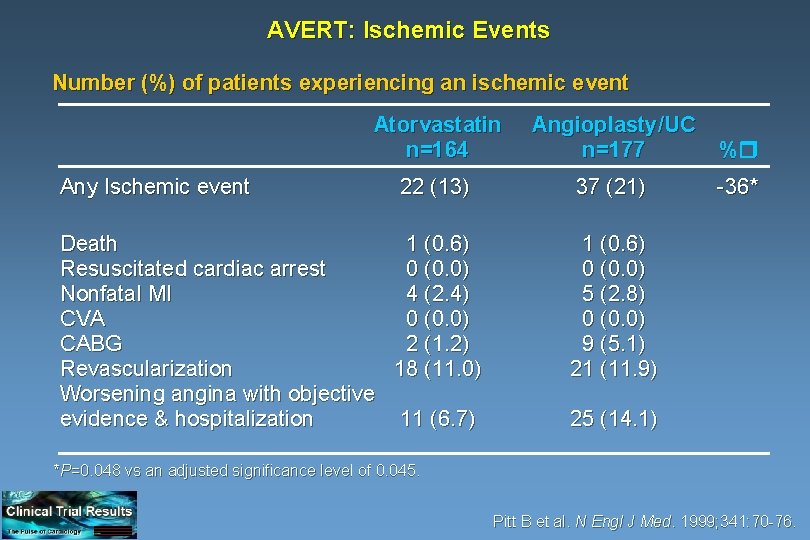
AVERT: Ischemic Events Number (%) of patients experiencing an ischemic event Any Ischemic event Atorvastatin n=164 Angioplasty/UC n=177 % 22 (13) 37 (21) -36* Death 1 (0. 6) Resuscitated cardiac arrest 0 (0. 0) Nonfatal MI 4 (2. 4) CVA 0 (0. 0) CABG 2 (1. 2) Revascularization 18 (11. 0) Worsening angina with objective evidence & hospitalization 11 (6. 7) 1 (0. 6) 0 (0. 0) 5 (2. 8) 0 (0. 0) 9 (5. 1) 21 (11. 9) 25 (14. 1) *P=0. 048 vs an adjusted significance level of 0. 045. Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

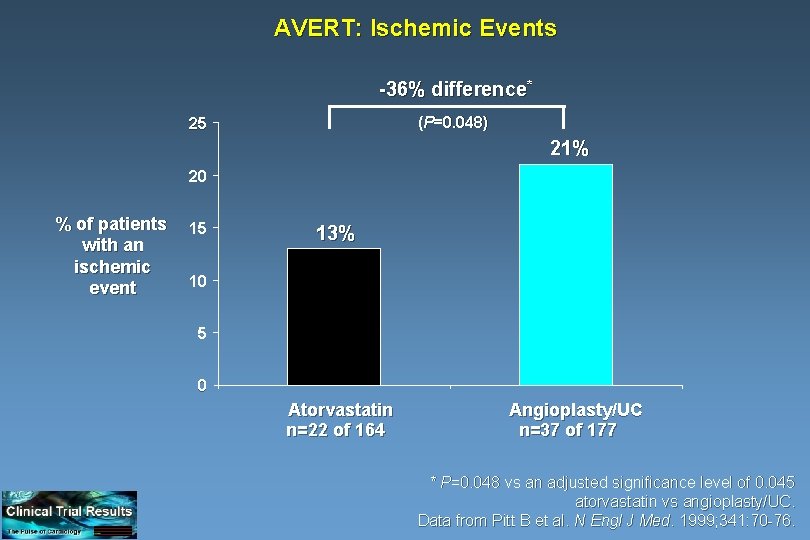
AVERT: Ischemic Events -36% difference* (P=0. 048) 25 21% 20 % of patients with an ischemic event 15 13% 10 5 0 Atorvastatin n=22 of 164 Angioplasty/UC n=37 of 177 * P=0. 048 vs an adjusted significance level of 0. 045 atorvastatin vs angioplasty/UC. Data from Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

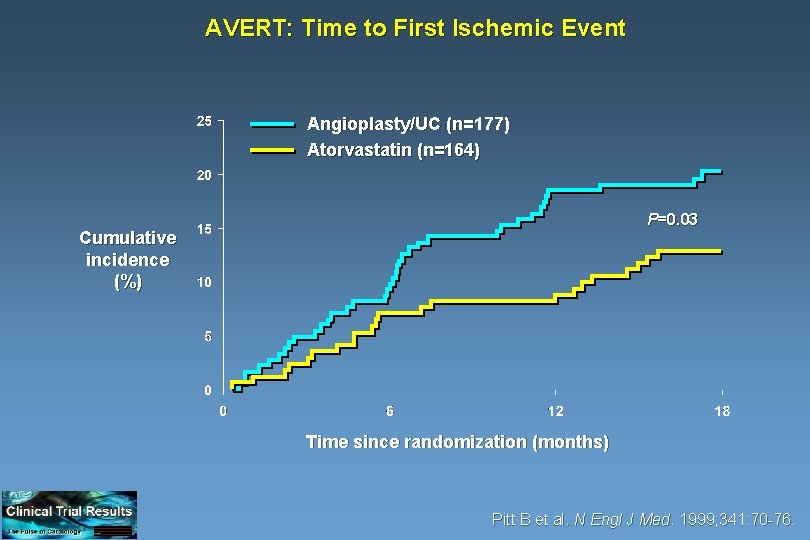
AVERT: Time to First Ischemic Event Angioplasty/UC (n=177) Atorvastatin (n=164) P=0. 03 Cumulative incidence (%) Time since randomization (months) Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

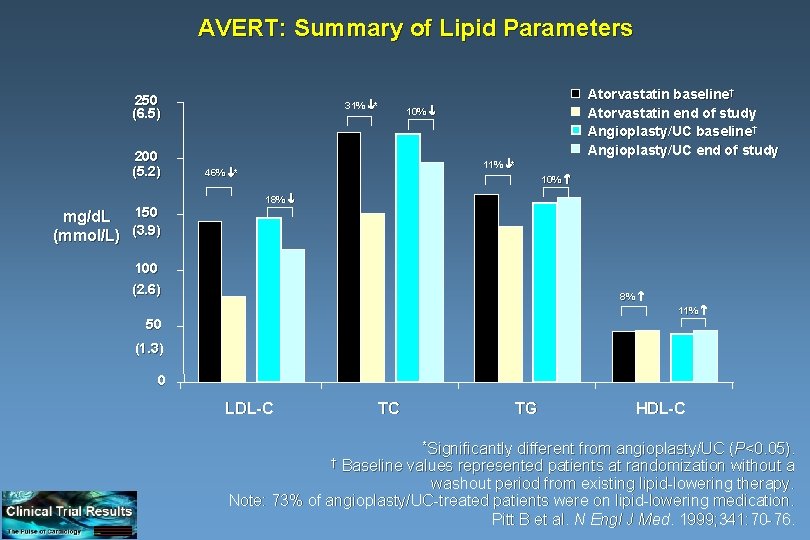
AVERT: Summary of Lipid Parameters 250 (6. 5) 200 (5. 2) mg/d. L 150 (mmol/L) (3. 9) 31% * Atorvastatin baseline† Atorvastatin end of study Angioplasty/UC baseline† Angioplasty/UC end of study 10% 11% * 46% * 10% 18% 100 (2. 6) 8% 11% 50 (1. 3) 0 LDL-C TC TG different from angioplasty/UC (P<0. 05). Baseline values represented patients at randomization without a washout period from existing lipid-lowering therapy. Note: 73% of angioplasty/UC-treated patients were on lipid-lowering medication. Pitt B et al. N Engl J Med. 1999; 341: 70 -76. † *Significantly HDL-C

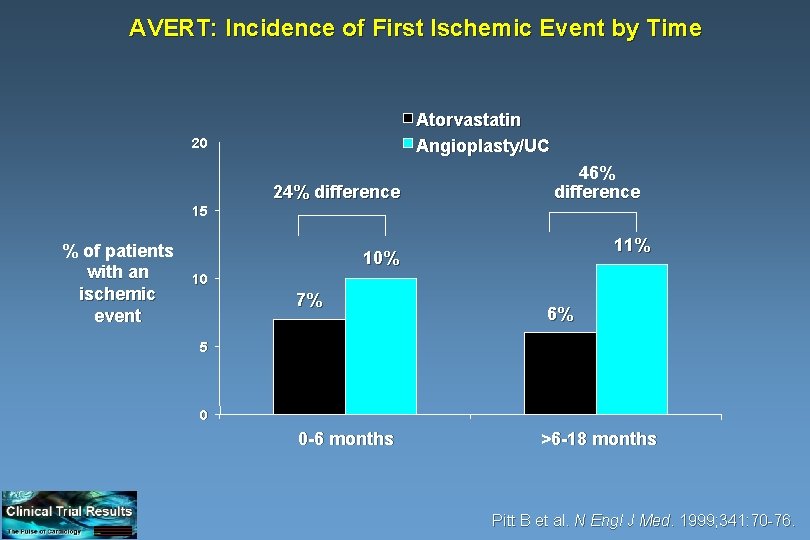
AVERT: Incidence of First Ischemic Event by Time Atorvastatin Angioplasty/UC 20 24% difference 46% difference 15 % of patients with an ischemic event 11% 10 7% 6% 5 0 0 -6 months >6 -18 months Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

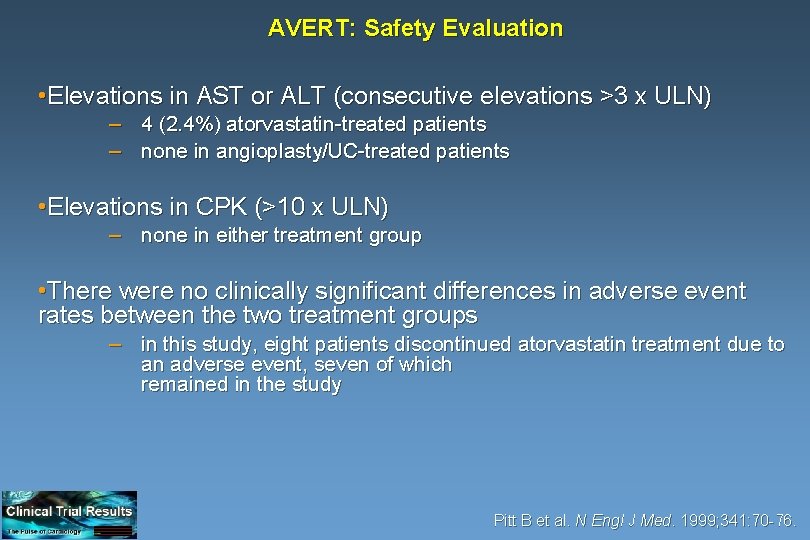
AVERT: Safety Evaluation • Elevations in AST or ALT (consecutive elevations >3 x ULN) – 4 (2. 4%) atorvastatin-treated patients – none in angioplasty/UC-treated patients • Elevations in CPK (>10 x ULN) – none in either treatment group • There were no clinically significant differences in adverse event rates between the two treatment groups – in this study, eight patients discontinued atorvastatin treatment due to an adverse event, seven of which remained in the study Pitt B et al. N Engl J Med. 1999; 341: 70 -76.

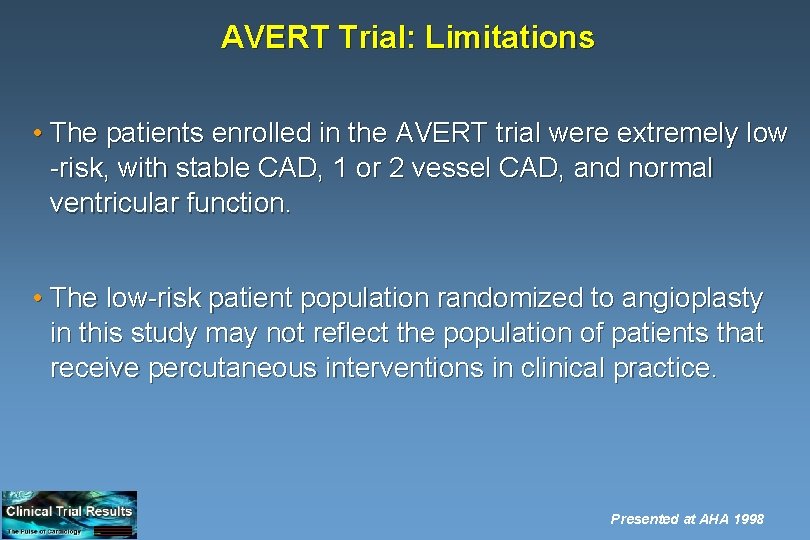
AVERT Trial: Limitations • The patients enrolled in the AVERT trial were extremely low -risk, with stable CAD, 1 or 2 vessel CAD, and normal ventricular function. • The low-risk patient population randomized to angioplasty in this study may not reflect the population of patients that receive percutaneous interventions in clinical practice. Presented at AHA 1998

AVERT Trial: Limitations (cont. ) • Although the primary endpoint was clinically interesting, it was not statistically significant after alpha adjustment for interim analyses. • Looking at the KM curves, it appears that the benefit of atorvastatin for ischemic events is not apparent until six months from the onset of therapy • It could be speculated that this is due to the time required for plaque stabilization and a reduction in new lesion development. Presented at AHA 1998

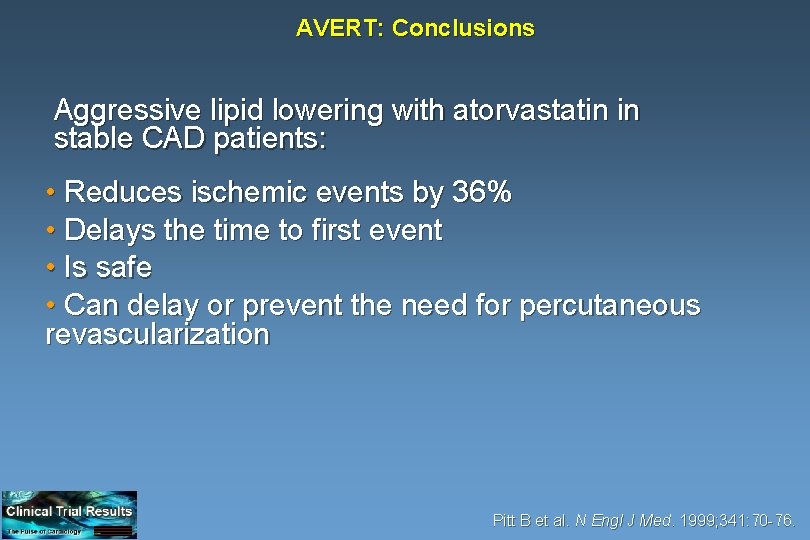
AVERT: Conclusions Aggressive lipid lowering with atorvastatin in stable CAD patients: • Reduces ischemic events by 36% • Delays the time to first event • Is safe • Can delay or prevent the need for percutaneous revascularization Pitt B et al. N Engl J Med. 1999; 341: 70 -76.
 What are the principal parts of verbs
What are the principal parts of verbs Rosuvastatin atorvastatin äquivalenz
Rosuvastatin atorvastatin äquivalenz Lovastatin weight loss
Lovastatin weight loss Caduet coupon
Caduet coupon Facial electrical treatments level 3
Facial electrical treatments level 3 Advanced surface treatments
Advanced surface treatments Multi element design
Multi element design Insomnia treatments
Insomnia treatments Hiv treatments
Hiv treatments Dementia treatments and interventions near patterson
Dementia treatments and interventions near patterson Treatments for acute renal failure
Treatments for acute renal failure Diabetes treatments
Diabetes treatments 1784-ktcx
1784-ktcx Content analysis system
Content analysis system High indulgence score
High indulgence score Malware versus virus
Malware versus virus Scenario of formal communication
Scenario of formal communication Tone v mood
Tone v mood