Transcarotid Artery Revascularization versus Transfemoral Carotid Artery Stenting




- Slides: 18

Transcarotid Artery Revascularization versus Transfemoral Carotid Artery Stenting for Treatment of Carotid Artery Stenosis Patric Liang, MD; Marc L. Schermerhorn, MD; Jens Eldrup-Jorgensen, MD; Jack L. Cronenwett, MD; Brian W. Nolan, MD; Vikram S. Kashyap, MD; Grace J. Wang, MD, MSCE; MD; Raghu L. Motaganahalli, MD; Mahmoud B. Malas, MD, MHS

Disclosures • MS is a consultant for Silk Road Medical, Medtronic, Endologix, Cook, and Abbott • VK is a National Co-PI for ROADSTERII. • RM is a consultant and proctor for Silk Road Medical. • MM is a site PI for ROADSTERI and ROADSTERII, and National PI for ROADSTERI long term follow-up study. • PL, JJ, JC, BN, and GW have no disclosures.

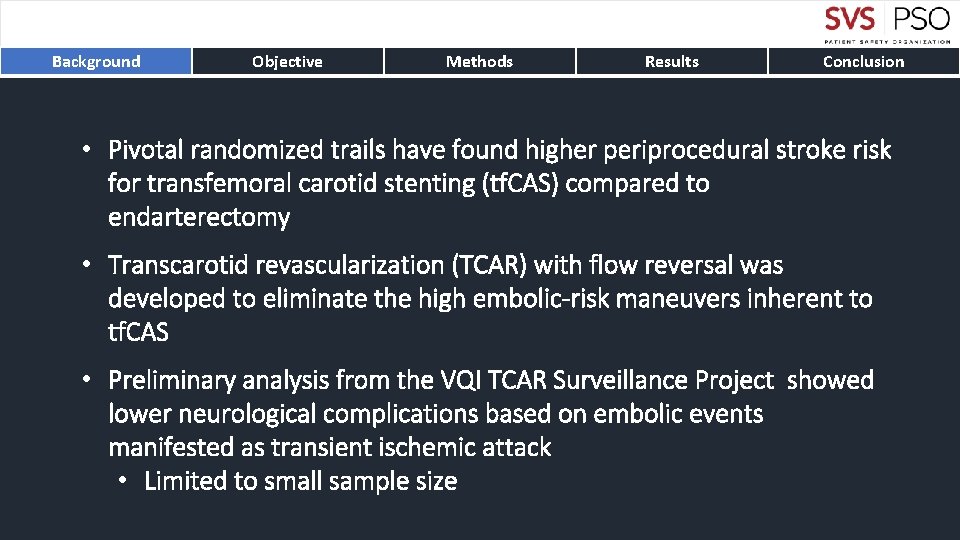
Background Objective Methods Results Conclusion • Pivotal randomized trails have found higher periprocedural stroke risk for transfemoral carotid stenting (tf. CAS) compared to endarterectomy • Transcarotid revascularization (TCAR) with flow reversal was developed to eliminate the high embolic-risk maneuvers inherent to tf. CAS • Preliminary analysis from the VQI TCAR Surveillance Project showed lower neurological complications based on embolic events manifested as transient ischemic attack • Limited to small sample size

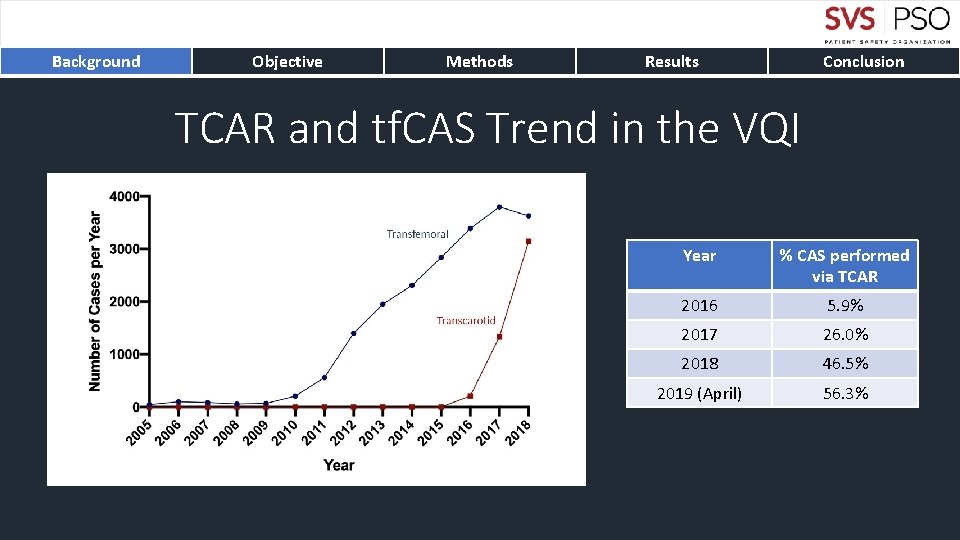
Background Objective Methods Results Conclusion TCAR and tf. CAS Trend in the VQI Year % CAS performed via TCAR 2016 5. 9% 2017 26. 0% 2018 46. 5% 2019 (April) 56. 3%

Background Objective Methods Results Conclusion Examine perioperative and one-year outcomes of patients undergoing TCAR and tf. CAS in the VQI TCAR Surveillance Project.

Background • • • Objective Methods Results Conclusion Prospective registry, clinical trial (NCT 02850588) • Study Period: September 2016 to April 2019 Inclusion • TCAR and tf. CAS procedures for atherosclerotic or intimal hyperplasic disease Exclusion – • Concomitant planned intracranial procedures • Unknown presenting symptom status or presenting symptom severity Primary Outcome • In-hospital, 30 -day, and 1 -year stroke/death Secondary Outcomes • Stroke, death, myocardial infarction, bleeding complication, procedure time, fluoroscopy time, contrast volume, CMS discharge criteria (failed discharge home or LOS >2 days) Propensity Score Matched Analysis

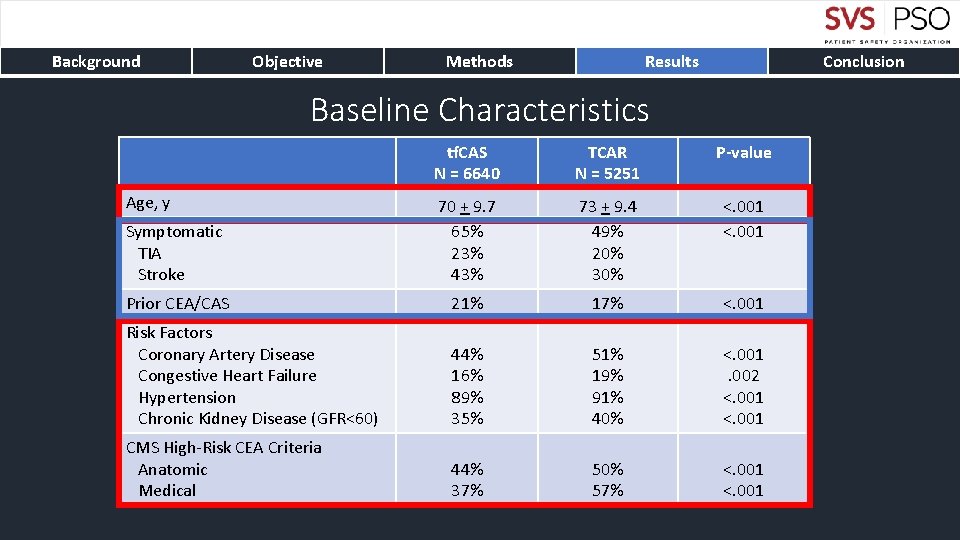
Background Objective Methods Results Conclusion Baseline Characteristics tf. CAS N = 6640 TCAR N = 5251 P-value Symptomatic TIA Stroke 70 + 9. 7 65% 23% 43% 73 + 9. 4 49% 20% 30% <. 001 Prior CEA/CAS 21% 17% <. 001 Risk Factors Coronary Artery Disease Congestive Heart Failure Hypertension Chronic Kidney Disease (GFR<60) 44% 16% 89% 35% 51% 19% 91% 40% <. 001. 002 <. 001 CMS High-Risk CEA Criteria Anatomic Medical 44% 37% 50% 57% <. 001 Age, y

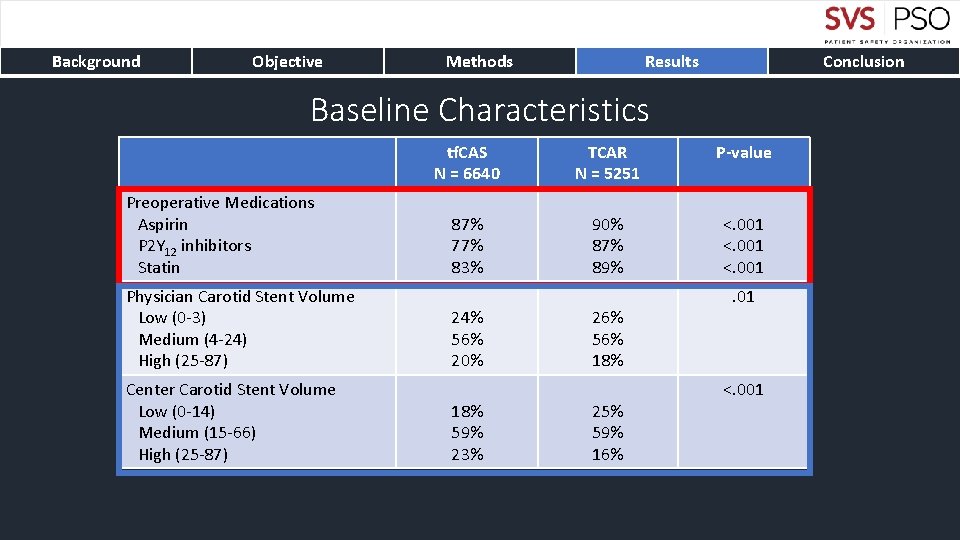
Background Objective Methods Results Conclusion Baseline Characteristics Preoperative Medications Aspirin P 2 Y 12 inhibitors Statin Physician Carotid Stent Volume Low (0 -3) Medium (4 -24) High (25 -87) Center Carotid Stent Volume Low (0 -14) Medium (15 -66) High (25 -87) tf. CAS N = 6640 TCAR N = 5251 P-value 87% 77% 83% 90% 87% 89% <. 001 24% 56% 20% 18% 59% 23% 26% 56% 18% 25% 59% 16% . 01 <. 001

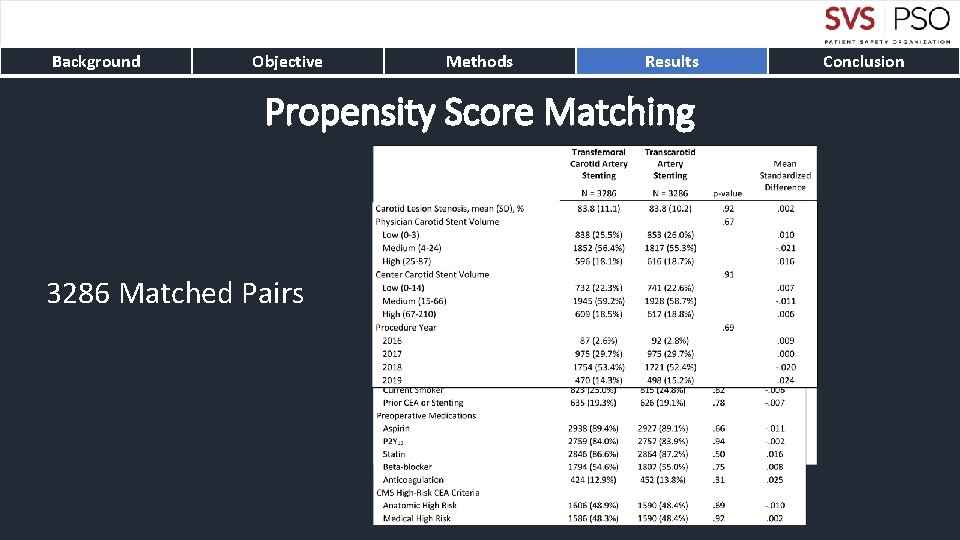
Background Objective Methods Results Propensity Score Matching 3286 Matched Pairs Conclusion

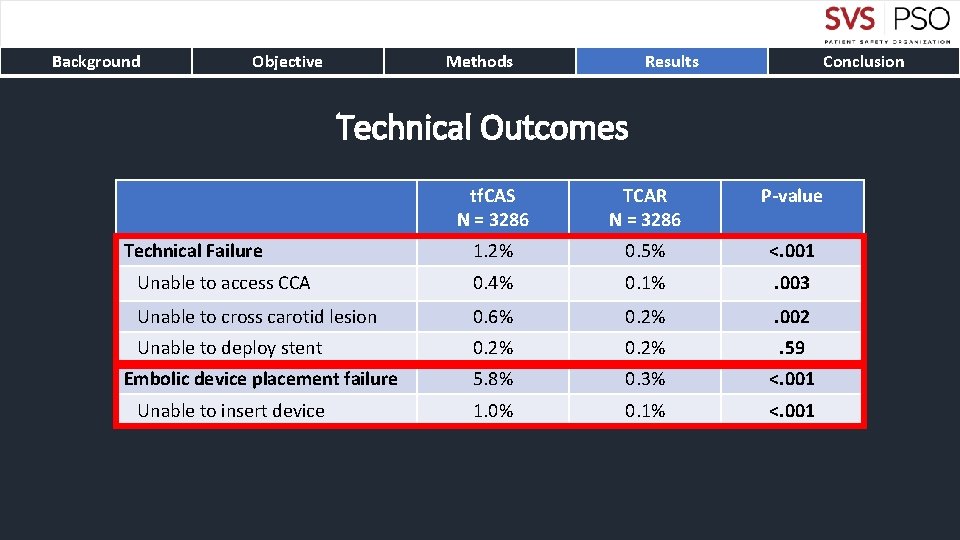
Background Objective Methods Results Conclusion Technical Outcomes tf. CAS N = 3286 TCAR N = 3286 P-value 1. 2% 0. 5% <. 001 Unable to access CCA 0. 4% 0. 1% . 003 Unable to cross carotid lesion 0. 6% 0. 2% . 002 Unable to deploy stent 0. 2% . 59 5. 8% 0. 3% <. 001 1. 0% 0. 1% <. 001 Technical Failure Embolic device placement failure Unable to insert device

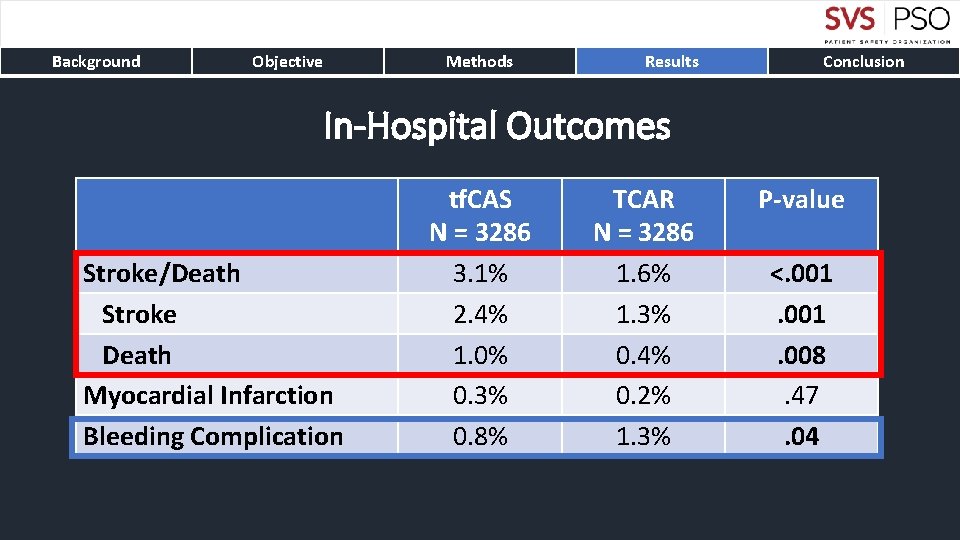
Background Objective Methods Results Conclusion In-Hospital Outcomes Stroke/Death Stroke Death Myocardial Infarction Bleeding Complication tf. CAS N = 3286 3. 1% 2. 4% 1. 0% 0. 3% 0. 8% TCAR N = 3286 1. 6% 1. 3% 0. 4% 0. 2% 1. 3% P-value <. 001. 008. 47. 04

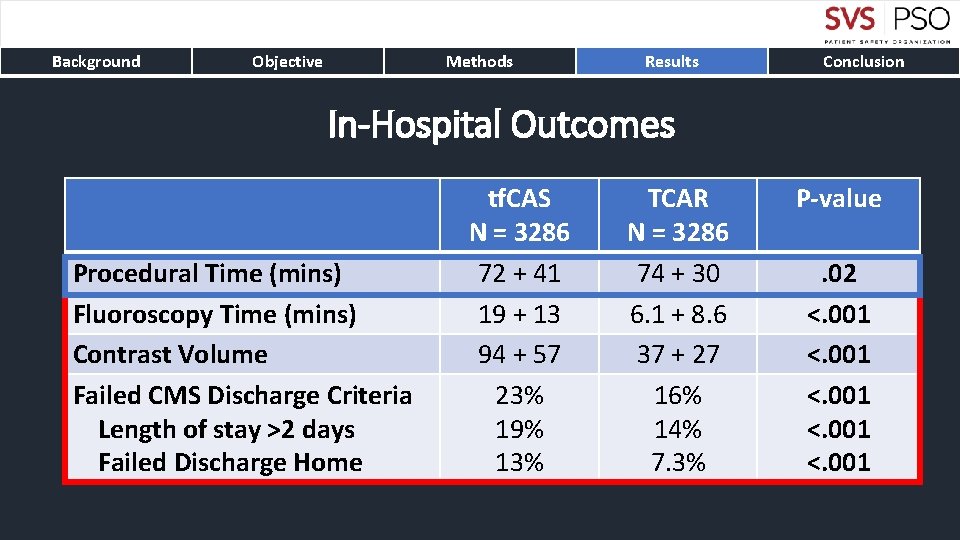
Background Objective Methods Results Conclusion In-Hospital Outcomes Procedural Time (mins) Fluoroscopy Time (mins) Contrast Volume Failed CMS Discharge Criteria Length of stay >2 days Failed Discharge Home tf. CAS N = 3286 72 + 41 19 + 13 94 + 57 23% 19% 13% TCAR N = 3286 74 + 30 6. 1 + 8. 6 37 + 27 16% 14% 7. 3% P-value. 02 <. 001

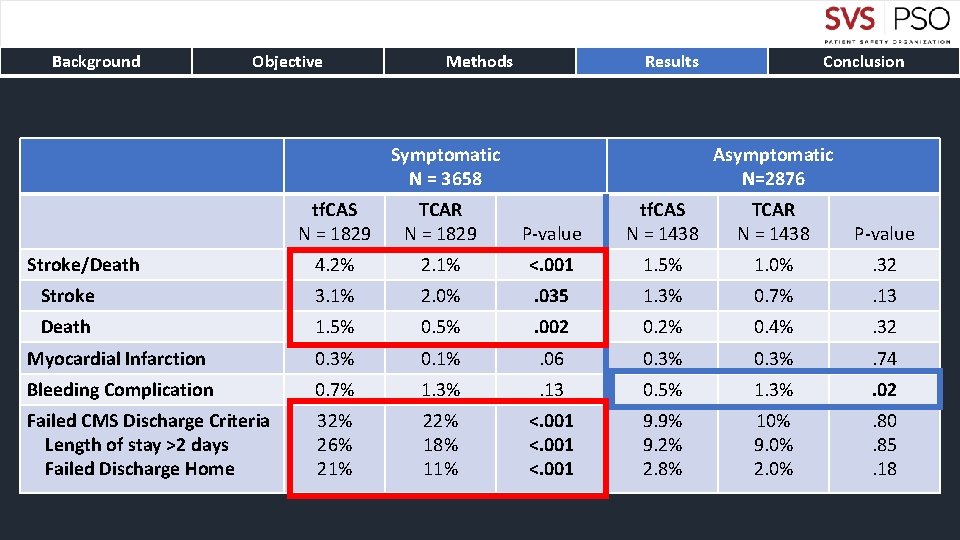
Background Objective Methods Results Symptomatic N = 3658 tf. CAS N = 1829 TCAR N = 1829 4. 2% Stroke Death Conclusion Asymptomatic N=2876 P-value tf. CAS N = 1438 TCAR N = 1438 P-value 2. 1% <. 001 1. 5% 1. 0% . 32 3. 1% 2. 0% . 035 1. 3% 0. 7% . 13 1. 5% 0. 5% . 002 0. 2% 0. 4% . 32 Myocardial Infarction 0. 3% 0. 1% . 06 0. 3% . 74 Bleeding Complication 0. 7% 1. 3% . 13 0. 5% 1. 3% . 02 Failed CMS Discharge Criteria Length of stay >2 days Failed Discharge Home 32% 26% 21% 22% 18% 11% <. 001 9. 9% 9. 2% 2. 8% 10% 9. 0% 2. 0% . 80. 85. 18 Stroke/Death

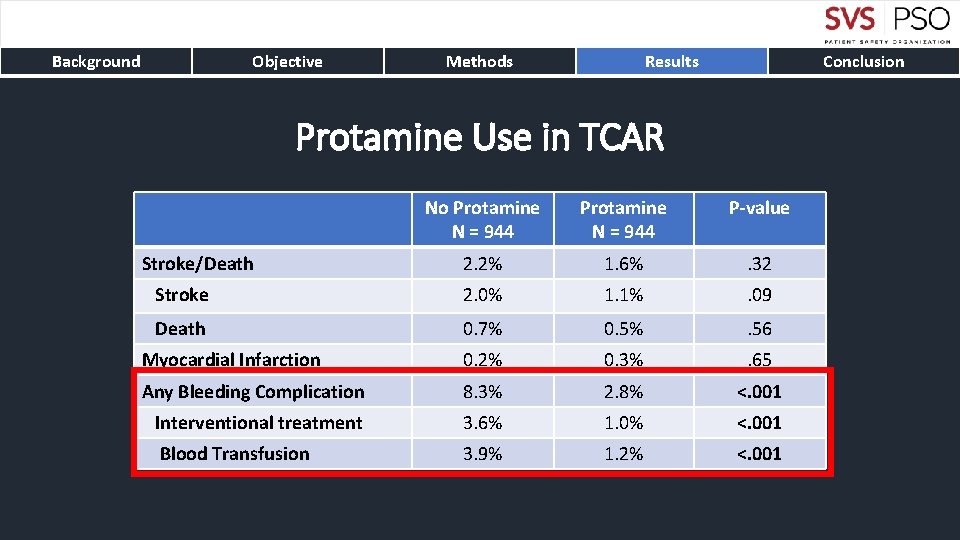
Background Objective Methods Results Conclusion Protamine Use in TCAR No Protamine N = 944 P-value 2. 2% 1. 6% . 32 Stroke 2. 0% 1. 1% . 09 Death 0. 7% 0. 5% . 56 Myocardial Infarction 0. 2% 0. 3% . 65 Any Bleeding Complication 8. 3% 2. 8% <. 001 Interventional treatment 3. 6% 1. 0% <. 001 Blood Transfusion 3. 9% 1. 2% <. 001 Stroke/Death

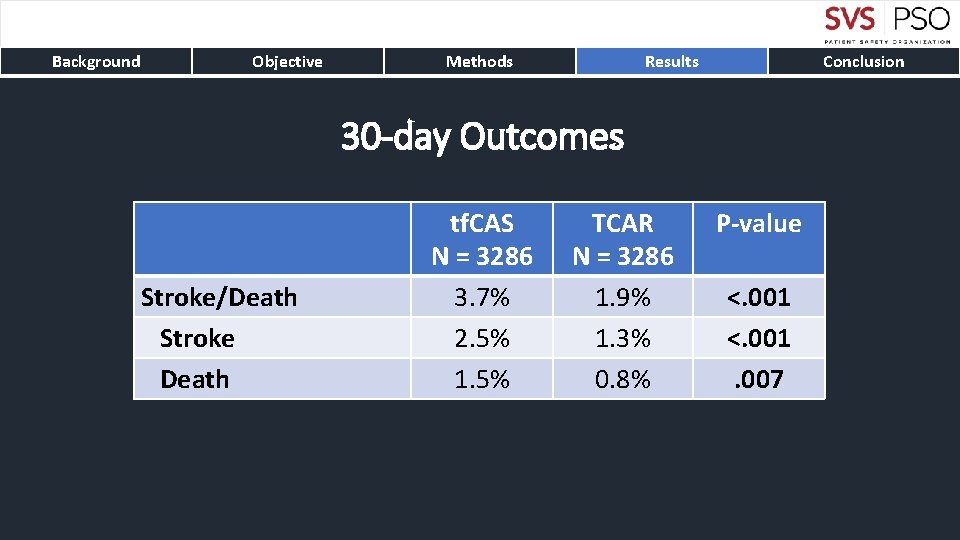
Background Objective Methods Results Conclusion 30 -day Outcomes Stroke/Death Stroke Death tf. CAS N = 3286 3. 7% 2. 5% 1. 5% TCAR N = 3286 1. 9% 1. 3% 0. 8% P-value <. 001. 007

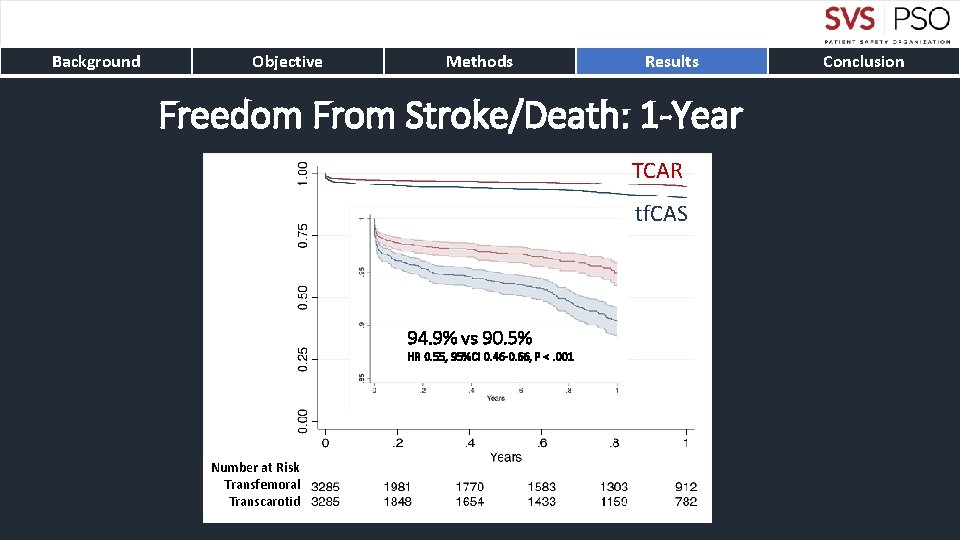
Background Objective Methods Results Freedom From Stroke/Death: 1 -Year TCAR tf. CAS 94. 9% vs 90. 5% HR 0. 55, 95%CI 0. 46 -0. 66, P <. 001 Number at Risk Transfemoral Transcarotid Conclusion

Background Objective Methods Results Conclusion • TCAR is associated with lower rates of stroke and death compared to tf. CAS • The stroke/death differences persistent up to one-year • Benefits from TCAR are particularly compelling for symptomatic carotid disease • Protamine use in TCAR results in significantly decreased bleeding complications without differences in thrombotic complications • TCAR with flow reversal should be preferred carotid stenting technique

 Ascending pharyngeal artery
Ascending pharyngeal artery Amaurosis fugax
Amaurosis fugax Retract defintion
Retract defintion Cat sinus anatomy
Cat sinus anatomy Brain arterial supply
Brain arterial supply External carotid artery branch
External carotid artery branch Blood vessel model
Blood vessel model Costocervical trunk
Costocervical trunk What artery supplies the hippocampus
What artery supplies the hippocampus Carotid node
Carotid node Carotid artery pulse
Carotid artery pulse Transfemoral gait deviations
Transfemoral gait deviations Iskial seki nedir
Iskial seki nedir Tap bifurcation technique
Tap bifurcation technique Mini culotte stenting
Mini culotte stenting Inverted provisional stenting
Inverted provisional stenting Superior mesenteric artery origin
Superior mesenteric artery origin Anterior inferior quadrant of tympanic membrane
Anterior inferior quadrant of tympanic membrane Carotid cana
Carotid cana