The Atoms Family Atoms are the smallest form










- Slides: 33

The Atoms Family Atoms are the smallest form of elements

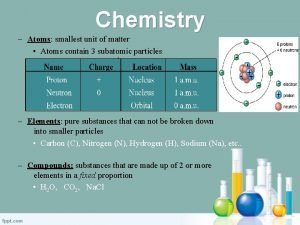
Matter • Anything that has mass and takes up space (has volume) • Made up of different kinds of atoms • Includes all things that can be seen, tasted, smelled, or touched • Does not include heat, sound, or light

In science, anything that has mass and takes up space is called matter Everything in the universe is made of matter A really loooooong time ago, the Greeks concluded that matter could be broken down into particles too small to be seen. They called these particles atoms

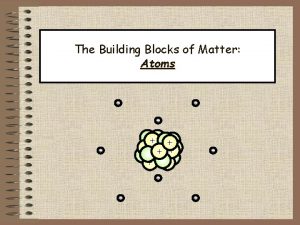
So, what’s an atom? An atom is the smallest piece that matter can be broken down to Okay, but I’ve never seen an atom. . . Where are they?

Atoms are EVERYWHERE!! plants hair desks boogers CDs Atoms are in. . . chocolate cheese hot dogs dirt air cars And even underwear

What do atoms look like? There is no "atomic microscope" which would allow one to look inside an atom and say, "Aha! There's 7 blue protons, 6 white neutrons, and 7 red electrons. " The way the structure of the atom was devised was through a long series of experiments Scientists have developed models. . .

Models • In the case of atoms, scientists use large models to explain something that is very small • Models of the atom were used to explain data or facts that were gathered experimentally. • So, these models are also theories

Early Models of the Atom Democritus • Universe was made of empty space and tiny bits of stuff • Called these tiny bits of stuff atomos • Atoms could not be divided

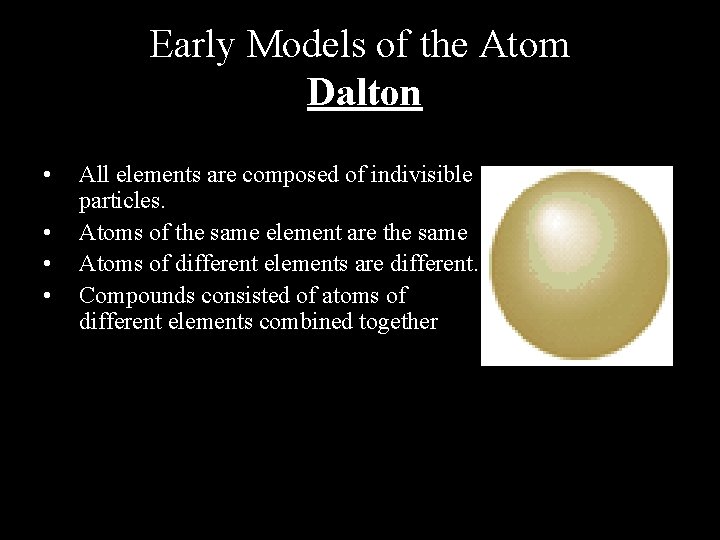
Early Models of the Atom Dalton • • All elements are composed of indivisible particles. Atoms of the same element are the same Atoms of different elements are different. Compounds consisted of atoms of different elements combined together

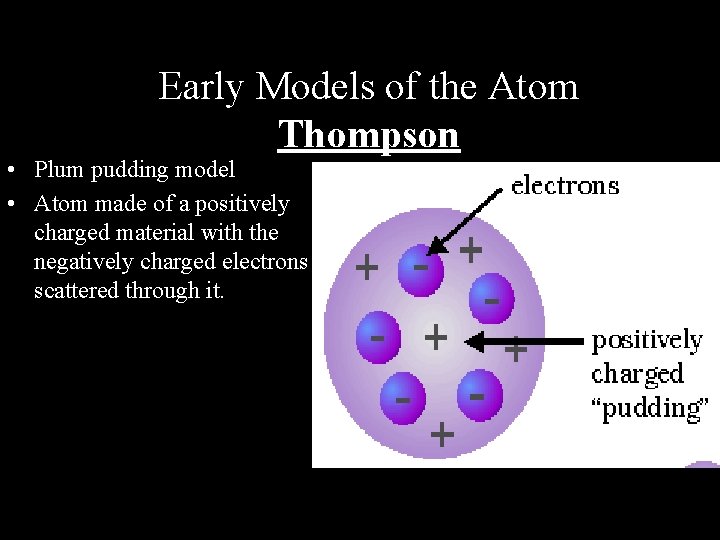
Early Models of the Atom Thompson • Plum pudding model • Atom made of a positively charged material with the negatively charged electrons scattered through it.

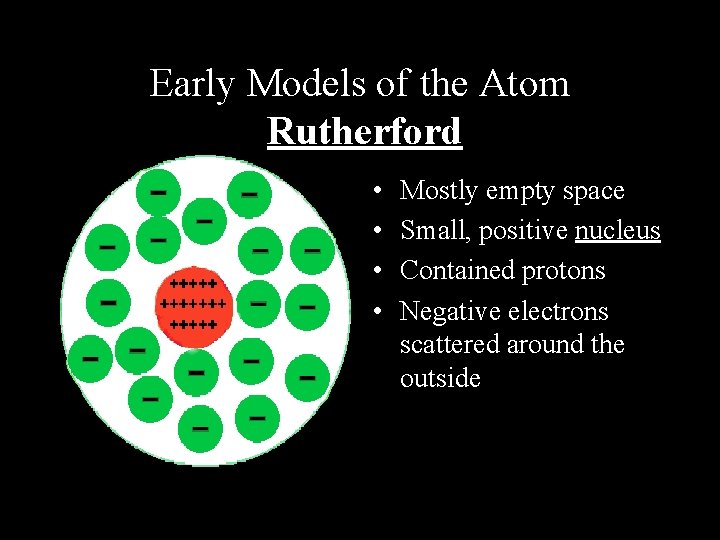
Early Models of the Atom Rutherford • • Mostly empty space Small, positive nucleus Contained protons Negative electrons scattered around the outside

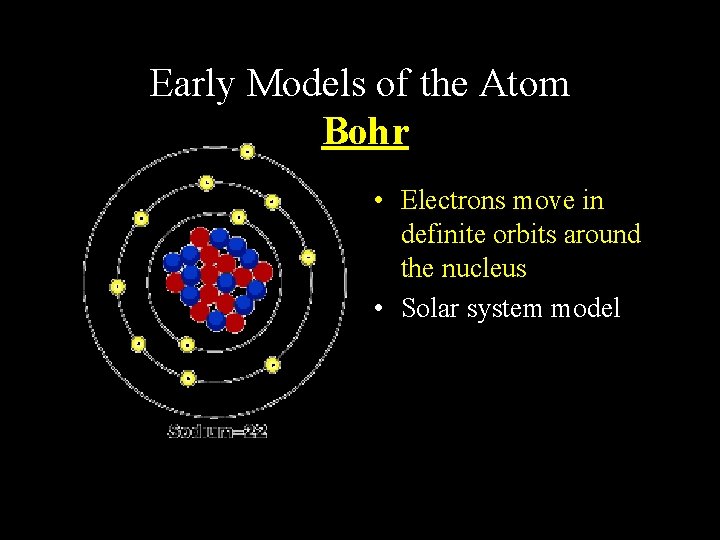
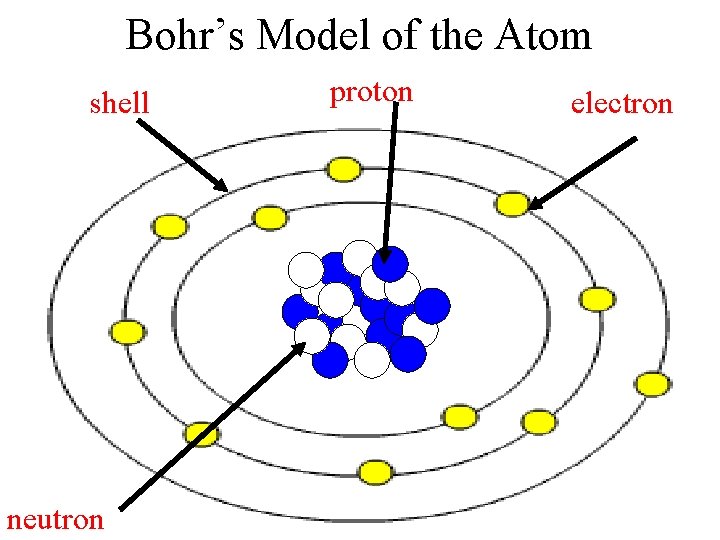
Early Models of the Atom Bohr • Electrons move in definite orbits around the nucleus • Solar system model

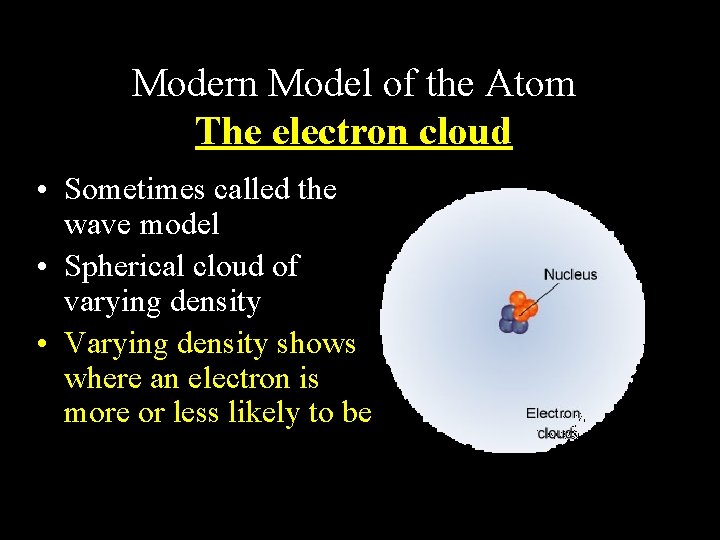
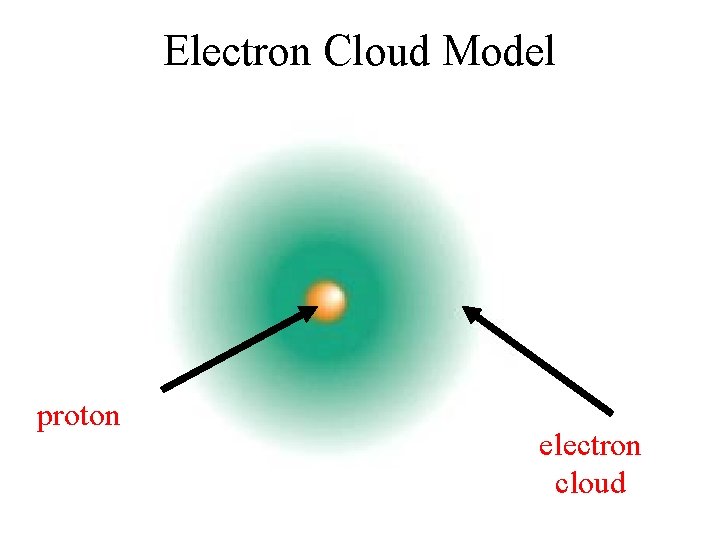
Modern Model of the Atom The electron cloud • Sometimes called the wave model • Spherical cloud of varying density • Varying density shows where an electron is more or less likely to be

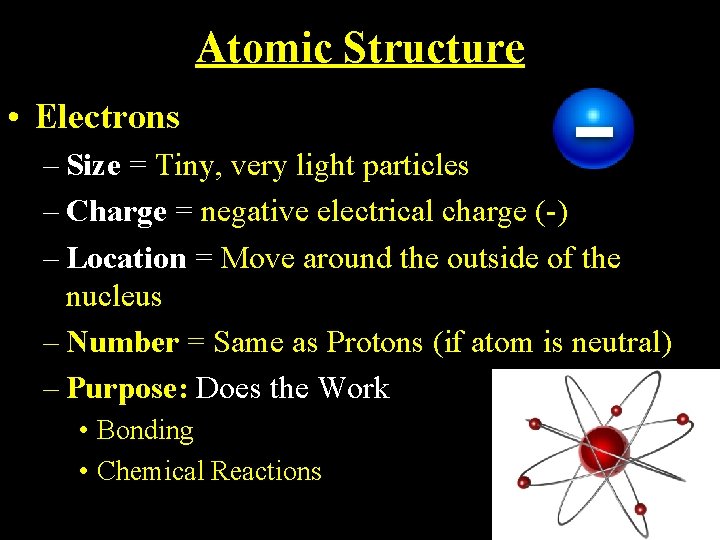
Atomic Structure • Electrons – Size = Tiny, very light particles – Charge = negative electrical charge (-) – Location = Move around the outside of the nucleus – Number = Same as Protons (if atom is neutral) – Purpose: Does the Work • Bonding • Chemical Reactions

Atomic Structure • Protons – Size = Much larger and heavier than electrons – Charge = positive charge (+) – Location = in nucleus of the atom – Number = Same as Atomic Number – Purpose: Keeps the electrons around

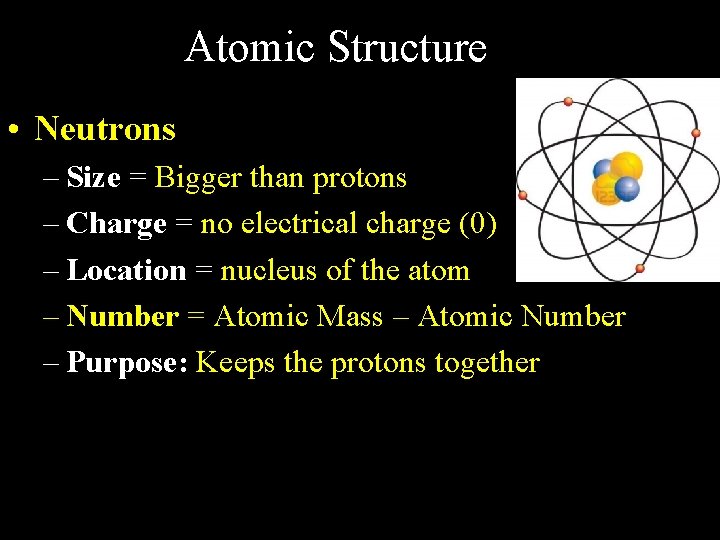
Atomic Structure • Neutrons – Size = Bigger than protons – Charge = no electrical charge (0) – Location = nucleus of the atom – Number = Atomic Mass – Atomic Number – Purpose: Keeps the protons together

Protons and Neutrons Protons and neutrons are responsible for most of the mass of an atom. Protons and neutrons are located in the nucleus of the atom

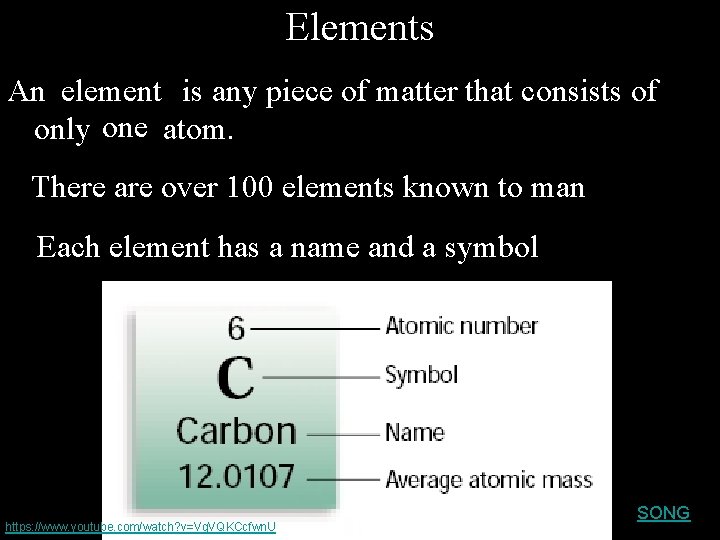
Elements An element is any piece of matter that consists of only one atom. There are over 100 elements known to man Each element has a name and a symbol https: //www. youtube. com/watch? v=Vg. VQKCcfwn. U SONG

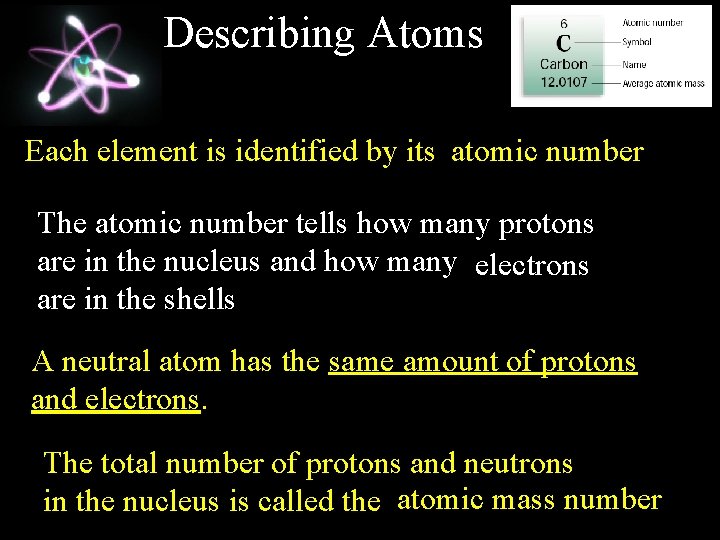
Describing Atoms Each element is identified by its atomic number The atomic number tells how many protons are in the nucleus and how many electrons are in the shells A neutral atom has the same amount of protons and electrons. The total number of protons and neutrons in the nucleus is called the atomic mass number

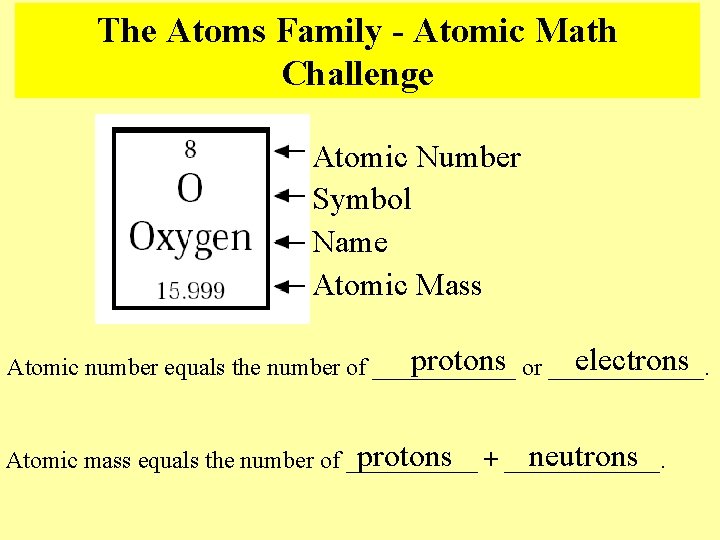
The Atoms Family - Atomic Math Challenge Atomic Number Symbol Name Atomic Mass electrons protons or _______. Atomic number equals the number of ______ protons + _______. neutrons Atomic mass equals the number of ______

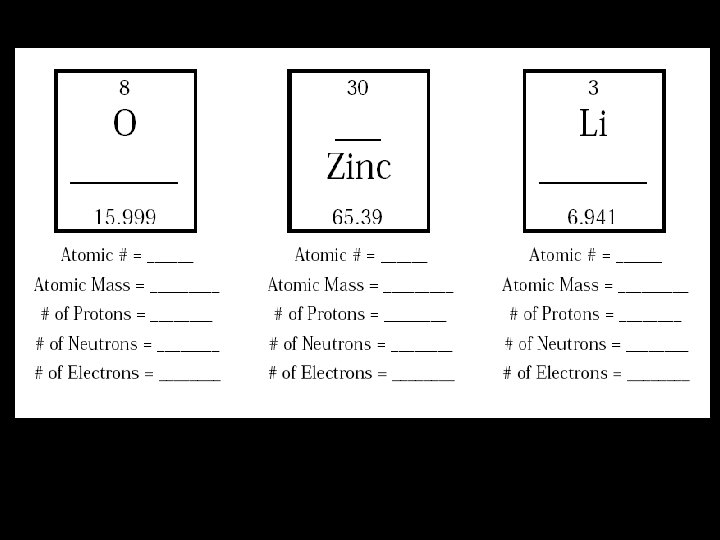
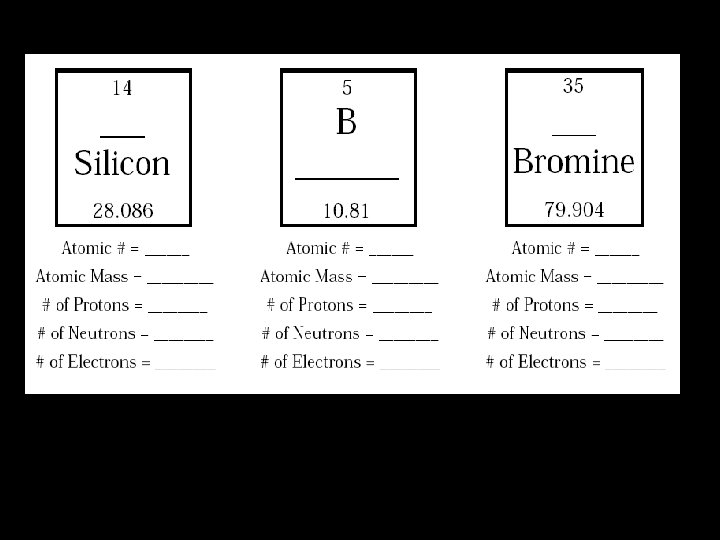
Atomic Math To determine the amount of neutrons for each element, subtract the atomic number from the atomic mass number How many protons does zinc have? 30 How many electrons does zinc have 30 How many neutrons does zinc have? 35


Assignment: Finish the rest of the worksheet and turn it in to your teacher.

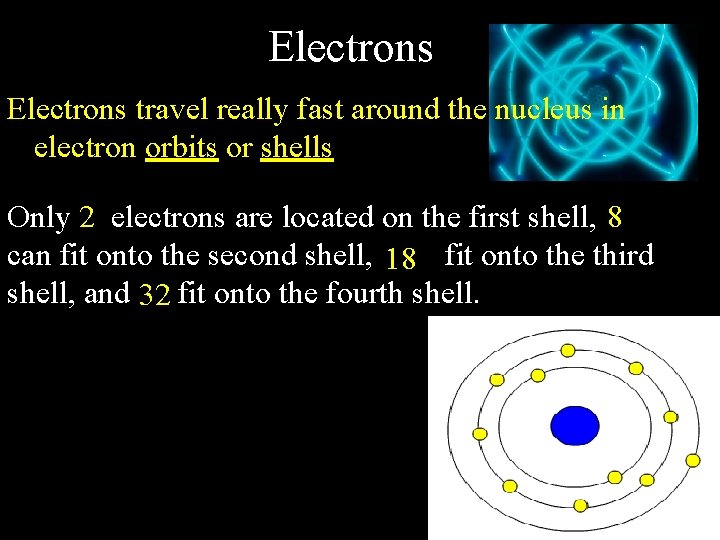
Electrons travel really fast around the nucleus in electron orbits or shells Only 2 electrons are located on the first shell, 8 can fit onto the second shell, 18 fit onto the third shell, and 32 fit onto the fourth shell.

Bohr’s Model of the Atom shell neutron proton electron

Electron Cloud Model proton electron cloud

Ions & Isotopes • The number of protons for a given atom never changes. The # of electrons and neutrons can change.

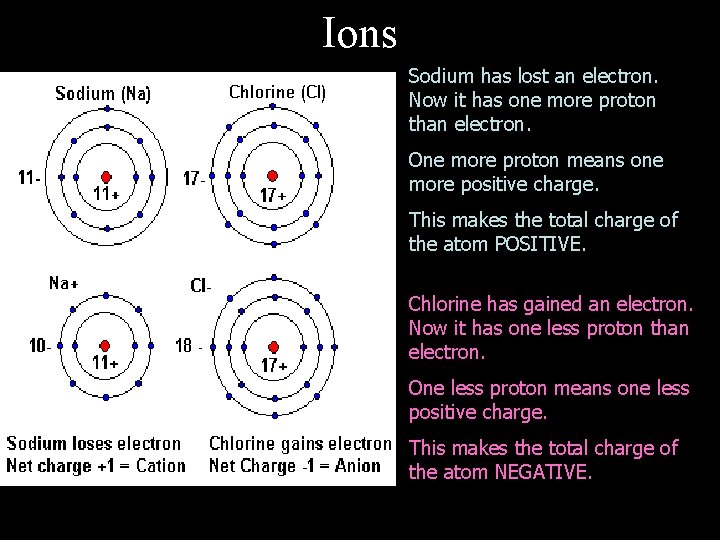
Ions • An atom that carries an electrical charge is called an ion • Cation – If the atom loses electrons, the atom becomes positively charged (because the number of positively charged protons will be more the number of electrons) • Anion – If an atom gains electrons, the atom becomes negatively charged (more negative charges than positive charges)

Ions Sodium has lost an electron. Now it has one more proton than electron. One more proton means one more positive charge. This makes the total charge of the atom POSITIVE. Chlorine has gained an electron. Now it has one less proton than electron. One less proton means one less positive charge. This makes the total charge of the atom NEGATIVE.

Isotopes • The number of protons for a given atom never changes. • The number of neutrons can change. • Atoms with different numbers of neutrons are called isotopes

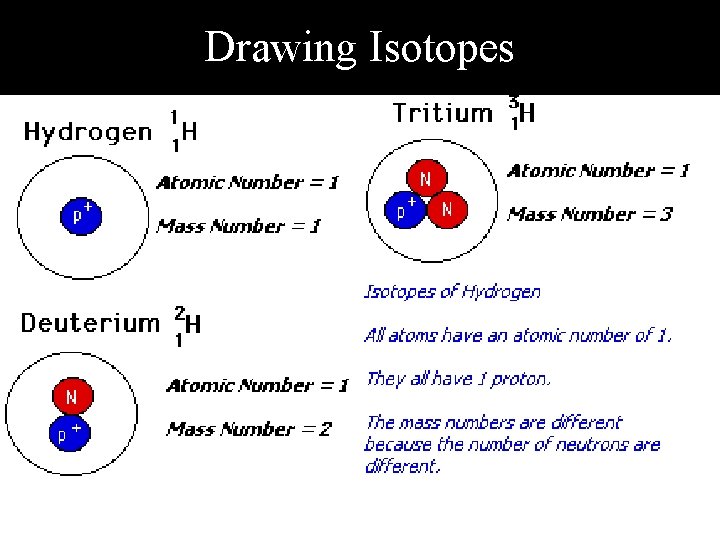
Drawing Isotopes

How to Write an isotope • Name of element – Mass Number (Protons + Neutrons) § Hydrogen – 1 (has one particle in the nucleus *the one particle in the nucleus is a proton § Hydrogen – 2 (has 2 particles in the nucleus) *Subtract the atomic number from the new atomic mass to get the new number of neutrons) § Hydrogen – 3 (has three particles in the nucleus) *Only one particle is a proton, the others must be nutrons)

Bill Nye Atoms • Study Jams Atoms: https: //www. youtube. com/watch? v=nm. GEh. Ulxn 2 M • Brainpop: – – Atoms: https: //www. brainpop. com/science/matterandchemistry/atoms/ Isotopes: https: //www. brainpop. com/science/matterandchemistry/isotopes/ Ions: https: //www. brainpop. com/science/matterandchemistry/ions/ Atomic Model: https: //www. brainpop. com/science/matterandchemistry/atomicmodel/ • Bill Nye Atoms: https: //www. schooltube. com/video/52 bb 23430 c 9 c 4 e 0 ebb 74/Bill%20 Nye%20%20 Atoms
 Insidan region jh
Insidan region jh Are atoms the smallest unit of matter
Are atoms the smallest unit of matter Smallest atoms in periodic table
Smallest atoms in periodic table The smallest building block of matter
The smallest building block of matter Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally The atoms family atomic math challenge answer key
The atoms family atomic math challenge answer key Matterville worksheet
Matterville worksheet The atom family atomic math challenge
The atom family atomic math challenge Nerdy nelda neutron
Nerdy nelda neutron Perky patty proton
Perky patty proton Matterville answer key
Matterville answer key The atoms family
The atoms family Atoms family worksheet
Atoms family worksheet Negative form present continuous
Negative form present continuous Whats ionic bond
Whats ionic bond Atoms form
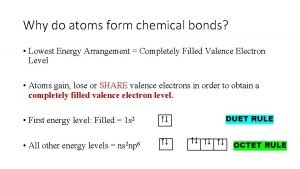
Atoms form Chemical bonds form when atoms *
Chemical bonds form when atoms * When atoms combine they form
When atoms combine they form Why do atoms form bonds
Why do atoms form bonds What atoms can have an expanded octet
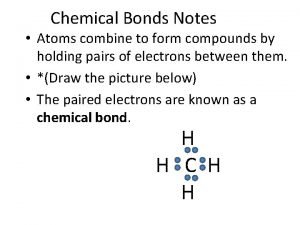
What atoms can have an expanded octet Do atoms combine to form compounds
Do atoms combine to form compounds Why do atoms form bonds
Why do atoms form bonds Atoms combine to form
Atoms combine to form Conclusion on topic family
Conclusion on topic family Carbon family
Carbon family Characteristics of single parent family
Characteristics of single parent family Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua