Atoms Atoms The smallest particle making up elements


![Isotopes ] Atoms of the same element but different mass number ] Boron-10 (10 Isotopes ] Atoms of the same element but different mass number ] Boron-10 (10](https://slidetodoc.com/presentation_image_h2/444c58f2b8d16762273baa1a287b7a9f/image-9.jpg)


- Slides: 14

Atoms

Atoms • • • The smallest particle making up elements Atoms consist of three types of particles Neutrons with a neutral charge Protons with a positive charge Electrons with a negative charge Mass is measured in a. m. u. (atomic mass unit)

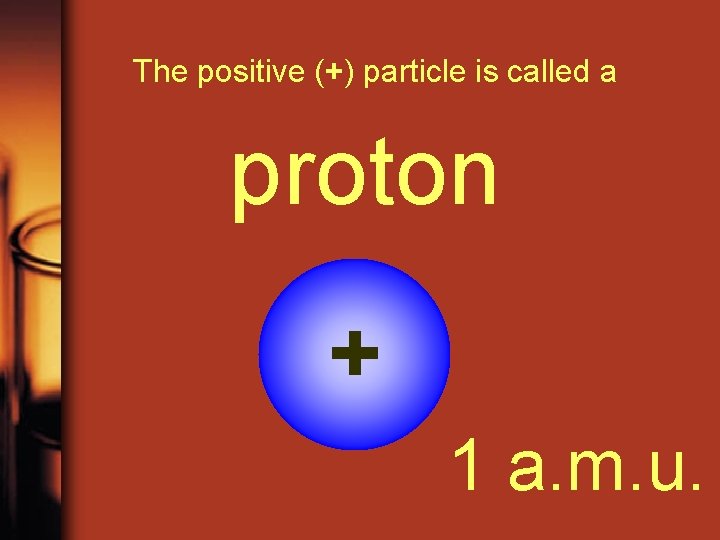
The positive (+) particle is called a proton + 1 a. m. u.

The neutral (no charge) particle is called a Neutron N 1 a. m. u.

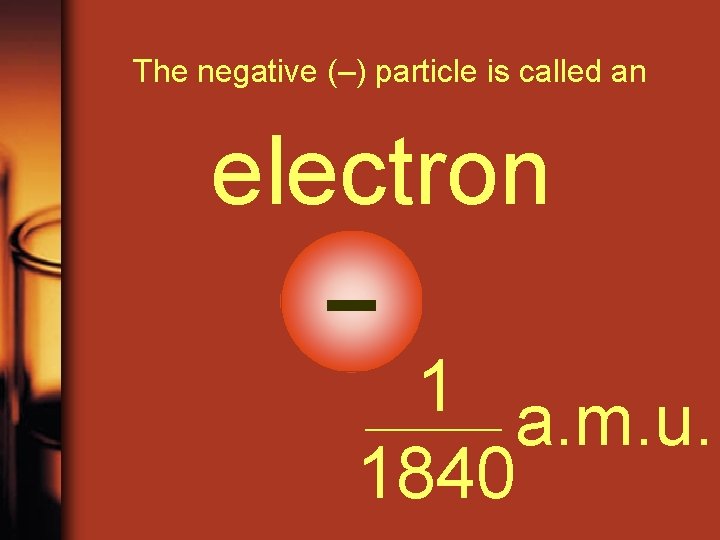
The negative (–) particle is called an electron – 1 a. m. u. 1840

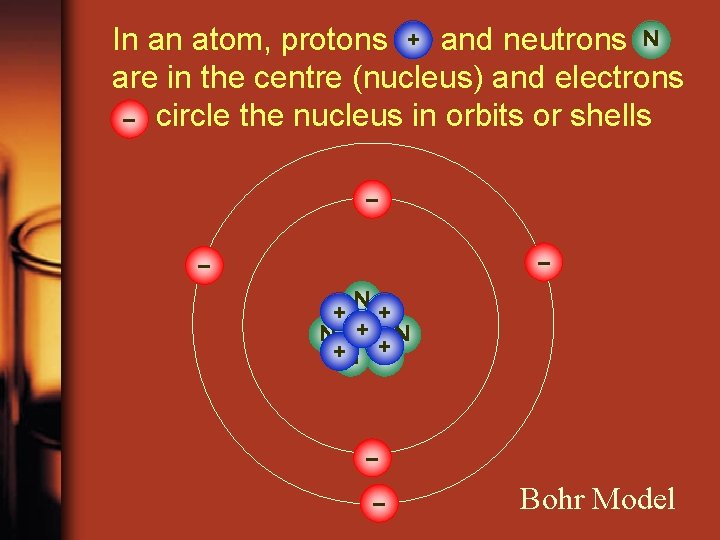
In an atom, protons + and neutrons N are in the centre (nucleus) and electrons – circle the nucleus in orbits or shells – – – +N+ N + +N +N N – – Bohr Model

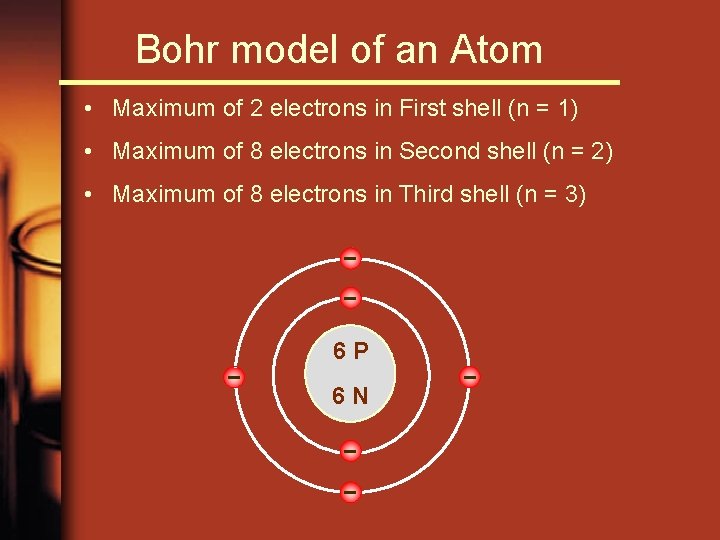
Bohr model of an Atom • Maximum of 2 electrons in First shell (n = 1) • Maximum of 8 electrons in Second shell (n = 2) • Maximum of 8 electrons in Third shell (n = 3) – – – 6 P 6 N – – –

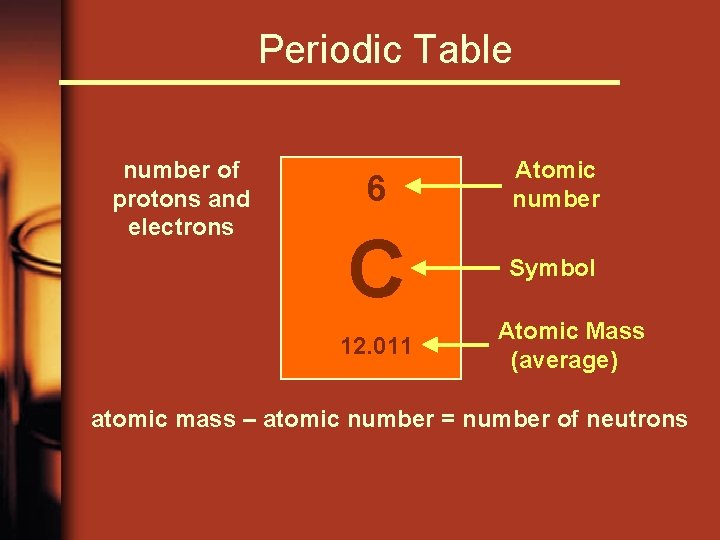
Periodic Table number of protons and electrons 6 Atomic number C Symbol 12. 011 Atomic Mass (average) atomic mass – atomic number = number of neutrons
![Isotopes Atoms of the same element but different mass number Boron10 10 Isotopes ] Atoms of the same element but different mass number ] Boron-10 (10](https://slidetodoc.com/presentation_image_h2/444c58f2b8d16762273baa1a287b7a9f/image-9.jpg)
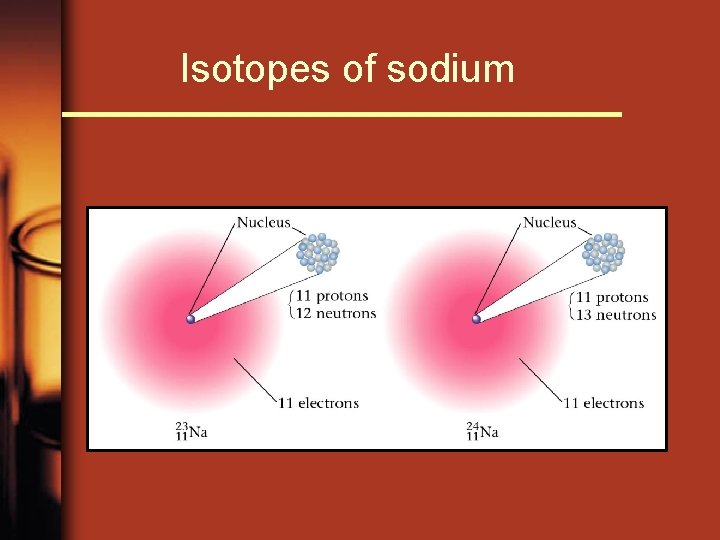
Isotopes ] Atoms of the same element but different mass number ] Boron-10 (10 B) has 5 p and 5 n ] Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

Isotopes of sodium

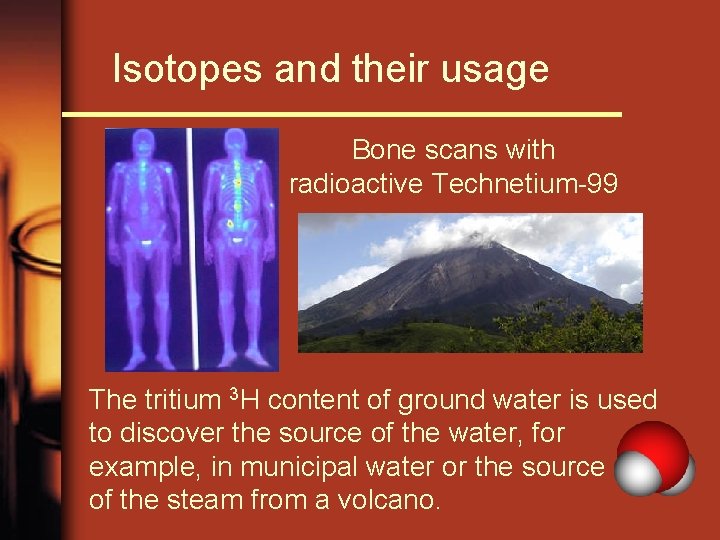
Isotopes and their usage Bone scans with radioactive Technetium-99 The tritium 3 H content of ground water is used to discover the source of the water, for example, in municipal water or the source of the steam from a volcano.

Counting particles • Protons: Atomic Number (from periodic table) • Neutrons: Mass Number minus the number of protons (mass number is protons and neutrons because the mass of electrons is negligible) • Electrons: – If it’s an atom, the protons and electrons must be the SAME so that it is has a net charge of zero (equal numbers of + and -) – If it does NOT have an equal number of electrons, it is not an atom, it is an ION.

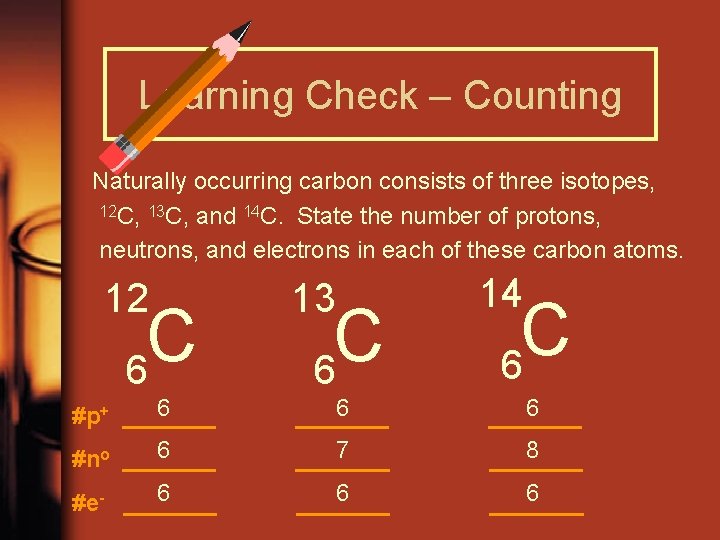
Learning Check – Counting Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12 C 6 13 C 6 14 C 6 6 #p+ _______ 6 #no _______ 6 _______ 7 _______ 6 _______ 8 _______ 6 #e- _______ 6 _______

Learning Check – Counting An atom has 14 protons and 20 neutrons. A. Its atomic number is 1) 14 2) 16 3) 34 B. Its mass number is 1) 14 2) 16 3) 34 C. The element is 1) Si 3) Se 2) Ca D. Another isotope of this element is 1) 34 X 2) 34 X 3) 36 X 16 14 14