Atomic Structure The Atoms Family Atom the smallest






- Slides: 21

Atomic Structure

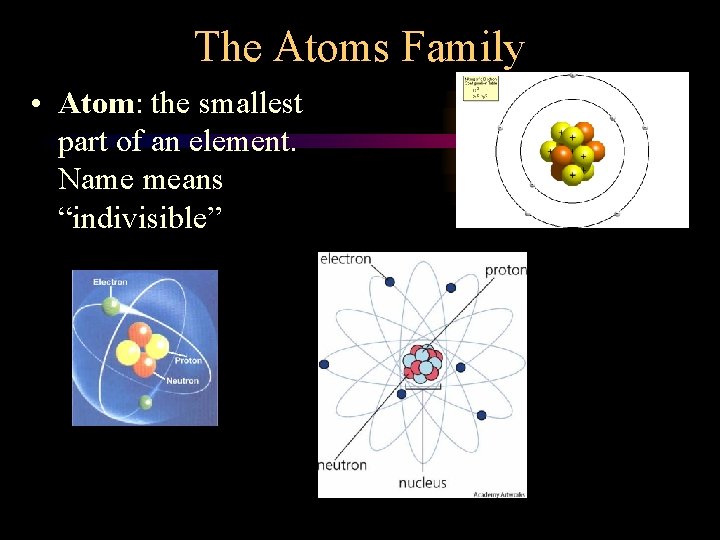
The Atoms Family • Atom: the smallest part of an element. Name means “indivisible”

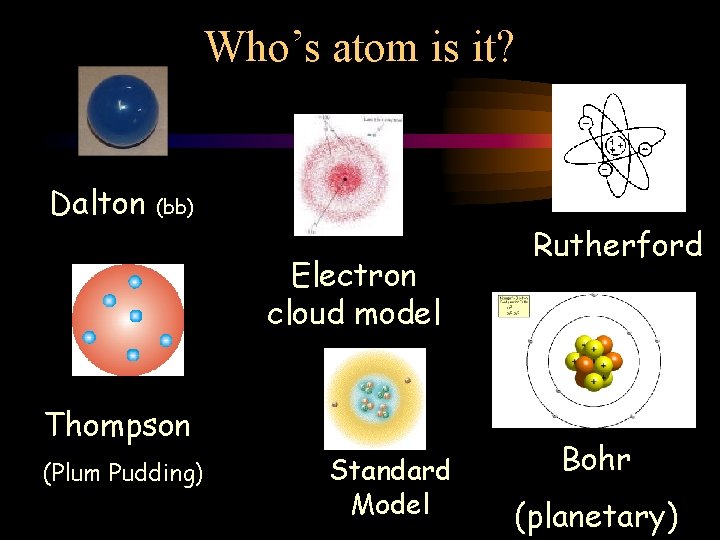
Who’s atom is it? Dalton (bb) Electron cloud model Thompson (Plum Pudding) Standard Model Rutherford Bohr (planetary)

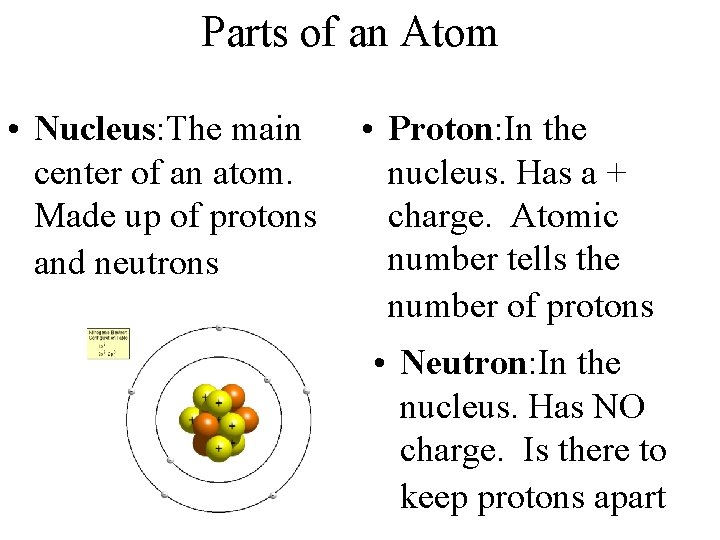
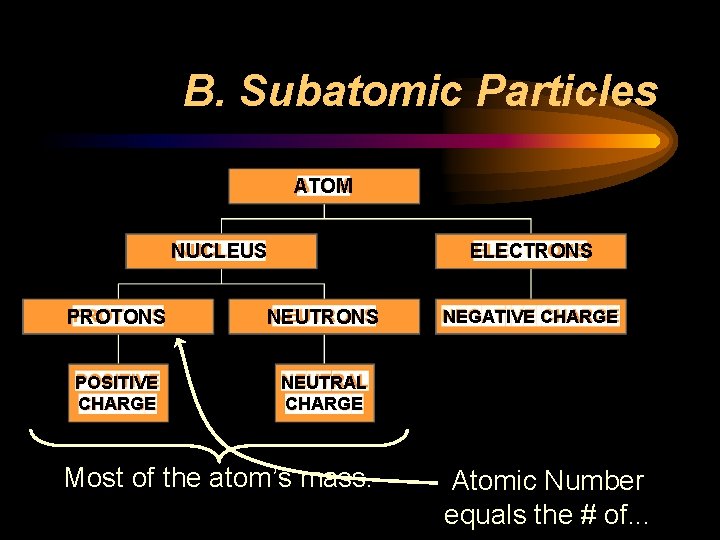
Parts of an Atom • Nucleus: The main center of an atom. Made up of protons and neutrons • Proton: In the nucleus. Has a + charge. Atomic number tells the number of protons • Neutron: In the nucleus. Has NO charge. Is there to keep protons apart

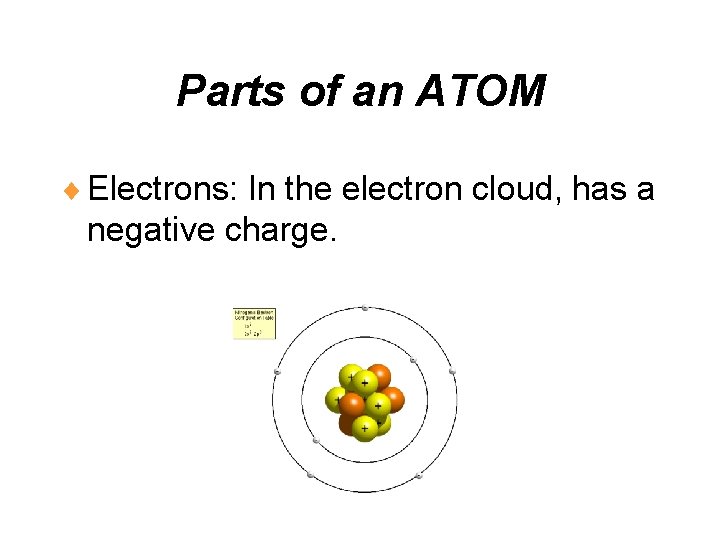
Parts of an ATOM ¨ Electrons: In the electron cloud, has a negative charge.

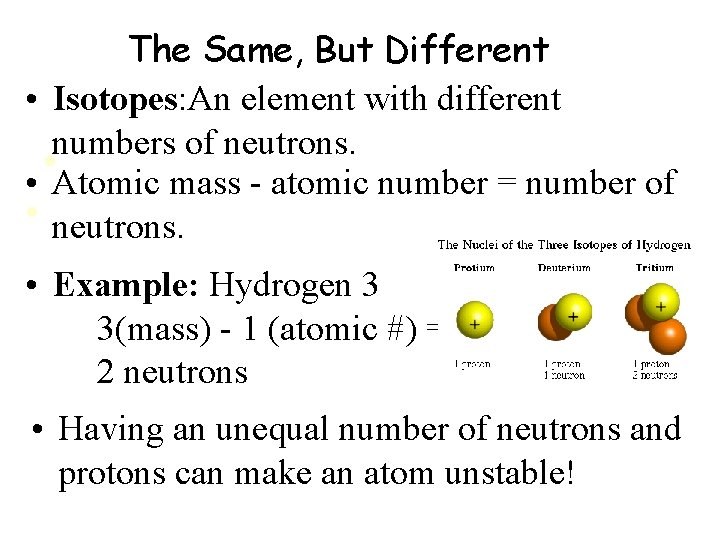
The Same, But Different • Isotopes: An element with different numbers of neutrons. • • Atomic mass - atomic number = number of • neutrons. • Example: Hydrogen 3 3(mass) - 1 (atomic #) = 2 neutrons • Having an unequal number of neutrons and protons can make an atom unstable!

B. Subatomic Particles ATOM NUCLEUS ELECTRONS PROTONS NEUTRONS POSITIVE CHARGE NEUTRAL CHARGE Most of the atom’s mass. NEGATIVE CHARGE Atomic Number equals the # of. . .

Let’s review • Name the two sub-atomic particles found in the nucleus of an atom: Proton and Neutron • What is the charge of a proton? Positive • What is the charge of a neutron? No charge (neutral) • Where are the electrons at? Orbiting the nucleus

Atomic Structure II. Electron Cloud Model ¨ Orbital ¨ Energy Levels ¨ Bohr Model Diagrams

Niels Bohr ¨ Bright-Line Spectrum (1913) • tried to explain presence of specific colors in hydrogen’s spectrum ¨ Energy Levels • electrons can only exist in specific energy states ¨ Planetary Model

Demonstration Time

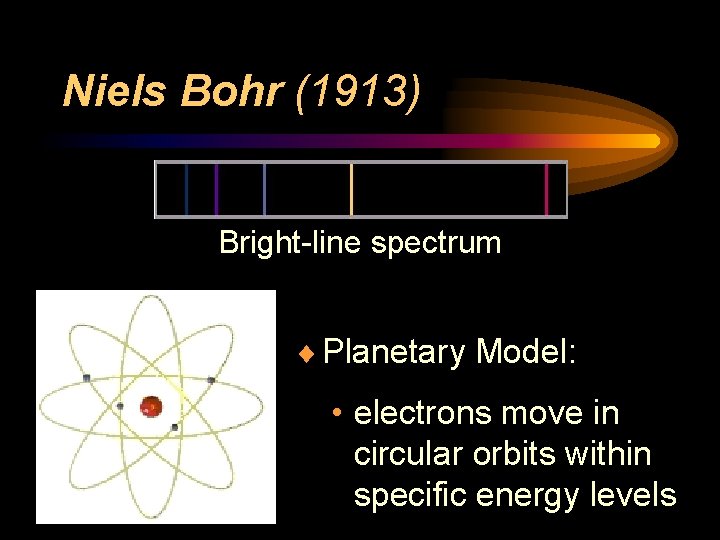
Niels Bohr (1913) Bright-line spectrum ¨ Planetary Model: • electrons move in circular orbits within specific energy levels

electron shells a) Electrons different amounts of energy and at different energy levels or electron shells. b) Electron shells (levels) determine… how an atom behaves when it encounters other atoms

Levels • 1 st Level: closest to nucleus. Has 1 orbital. Can hold 2 electrons • 2 nd level: next one out. Has 2 orbitals. Can hold 8 electrons. • 3 rd level: Holds 8 electrons. • 4 th level: Holds 18 electrons

Why are electrons important? 1) Elements have different electron configurations so different levels of bonding 2) Valence electrons are the electrons in the outer most shell. 3) Valence electrons are important because they affect how the element reacts with other elements.

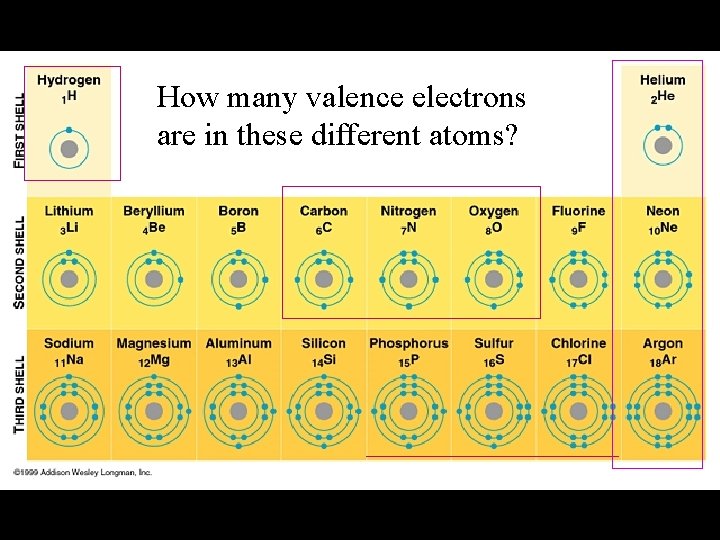
How many valence electrons are in these different atoms?

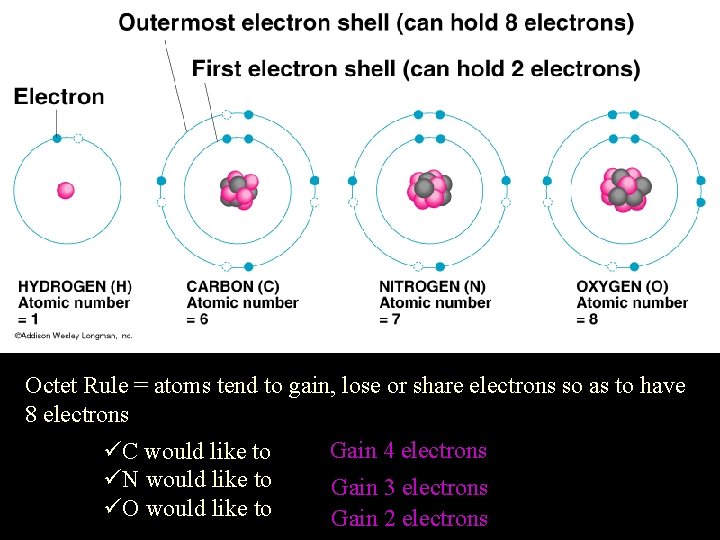
Octet Rule = atoms tend to gain, lose or share electrons so as to have 8 electrons üC would like to üN would like to üO would like to Gain 4 electrons Gain 3 electrons Gain 2 electrons

C. Stability ¨ Octet Rule • most atoms form bonds in order to have 8 valence e • full outer energy level Ne • like the Noble Gases! ¨ Stability is the driving force behind bond formation!

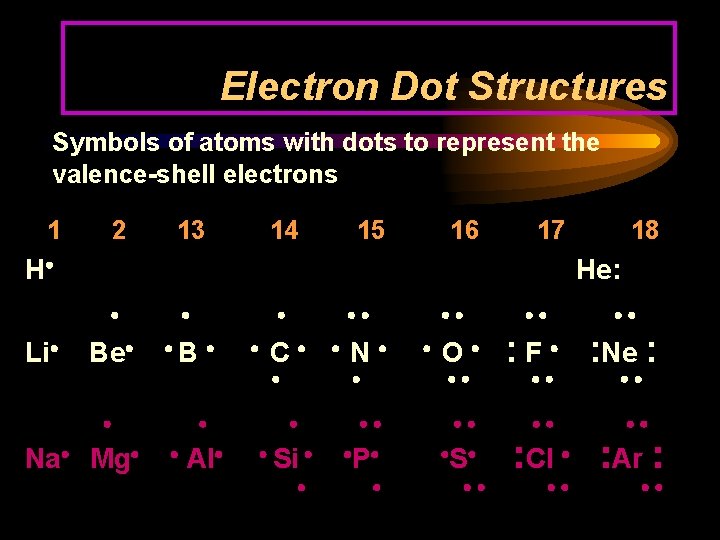
Electron Dot Structures Symbols of atoms with dots to represent the valence-shell electrons 1 2 13 14 15 16 17 H Li 18 He: Be B Na Mg Al C Si N O P S : F : Ne : : Cl : Ar :

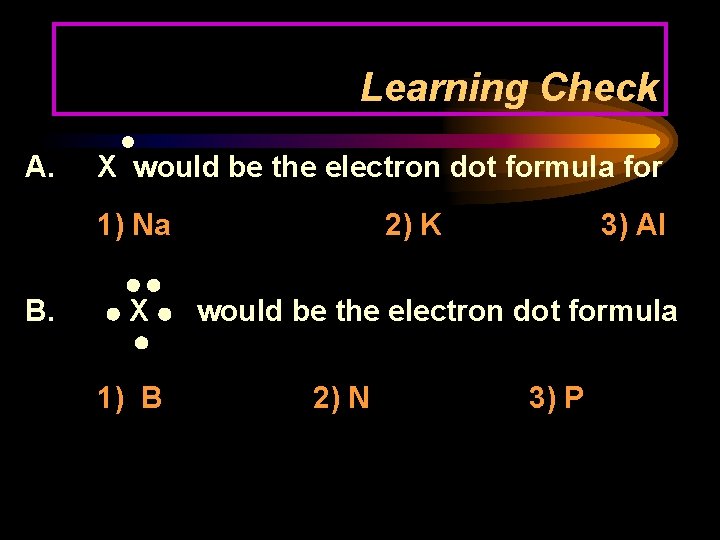
Learning Check A. X would be the electron dot formula for 1) Na B. X 1) B 2) K 3) Al would be the electron dot formula 2) N 3) P

Stability ¨ Why is it important for an atom to be “stable”? • So it is less reactive. ¨ Why are noble gases stable? • They have a full energy level.