THE STRUCTURE OF AN ATOM Atomic Structure Atoms









- Slides: 13

THE STRUCTURE OF AN ATOM

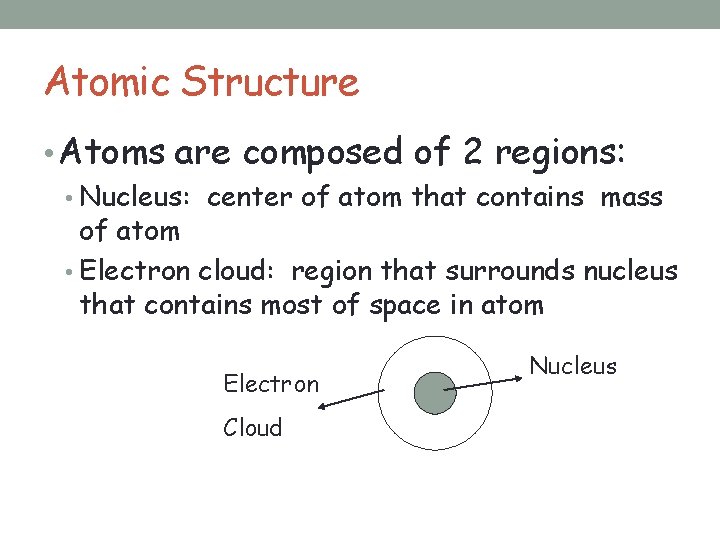
Atomic Structure • Atoms are composed of 2 regions: • Nucleus: center of atom that contains mass of atom • Electron cloud: region that surrounds nucleus that contains most of space in atom Electron Cloud Nucleus

What’s in the Nucleus? • Nucleus contains 2 of 3 subatomic particles: • Protons: subatomic particle w/ 1+ charge (p +) • Rutherford - 1911 • Neutrons: subatomic particle w/ no charge (no)

What’s in the Electron Cloud? • The 3 rd subatomic particle resides outside nucleus in electron cloud • Electron: subatomic particle w/ 1 - charge (e - ) and virtually no mass

How do these particles interact? • Protons and neutrons live compacted in tiny nucleus • most atom’s mass • electrons small and reside outside nucleus • small mass (2000 e- = 1 p+ or no) • occupy large volume of space outside nucleus

How do we know the number of subatomic particles in an atom? • Atomic #: indicates # of protons in atom • Ex: Hydrogen’s atomic # is 1 • hydrogen has 1 proton • Ex: Carbon’s atomic # is 6 • carbon has 6 protons **Number of protons identifies element similar to how your fingerprint ID’s you. Ex. 2 protons = He, 29 protons = Cu ALWAYS!!

How do we know the number of subatomic particles in an atom? • Mass number: number of protons and neutrons in nucleus (p+ + no) • Ex: hydrogen can have a mass # of 3. Since it has 1 proton it must have 2 neutrons • # of neutrons = mass # - atomic #

What are Isotopes? • Atoms of same element with different # of neutrons • Same atomic # • Different mass # (b/c neutrons are different) • Ex. Carbon 12, Carbon 13, and Carbon 14 all naturally occurring isotopes of Carbon. • Each has 6 p+ and 6 e-, but each has different # of neutrons (therefore, different mass#)

Determining the number of protons and neutrons • Li has mass # of 7 and atomic # of 3 • Protons = 3 (same as atomic #) • Neutrons= 7 -3 = 4 (mass # - atomic #) • Ne has a mass # of 20 and an atomic # of 10 • Protons = 10 • Neutrons = 20 - 10= 10

What about the electrons? • electrons are equal to protons • So e- = p+ = atomic # • Ex: He has mass # of 4 and atomic # of 2 • p+ = 2 • no = 2 • e- = 2 Basic Atomic Structure 1: 57

Determine the number of subatomic particles in the following: • Chlorine has a mass # of 35 and an atomic # of 17 • p+ = 17, no = 18, e- = 17 • Potassium has a mass # of 39 and an atomic # of 19 • P+ = 19, no = 20 e- = 19

Candy Atoms • Atom #1 - mass # of 5 and an atomic # of 3. • Atom #2 – 5 protons and 7 neutrons. • Atom #3 – Atomic # of 7 and 8 neutrons.

Candy Atoms • Atom #4 – mass # 18 and 9 electrons • Atom #5 – build your own candy atom using the candies that you have. You should be able to accurately determine: • Atomic # • Mass # • # of protons, neutrons, and electrons