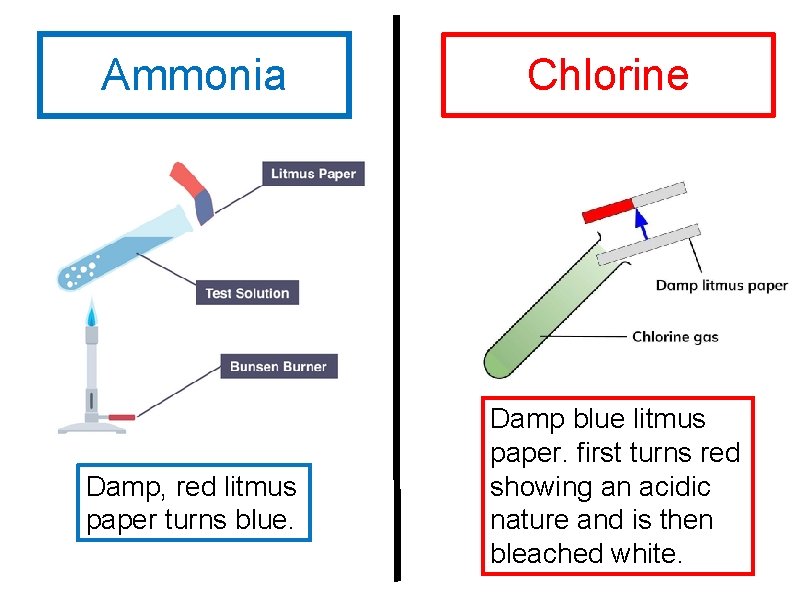
Testing for Gases Ammonia Damp red litmus paper





































- Slides: 58

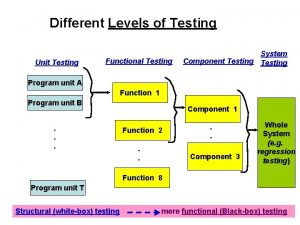
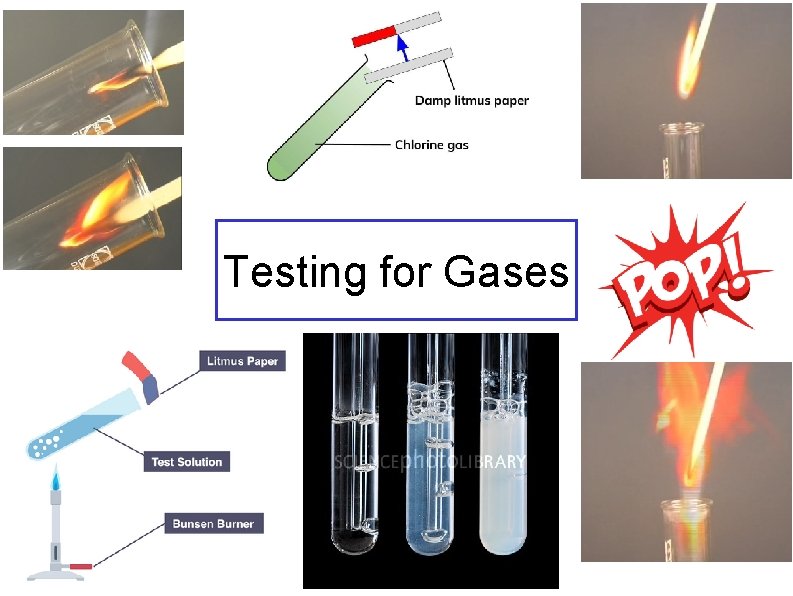
Testing for Gases

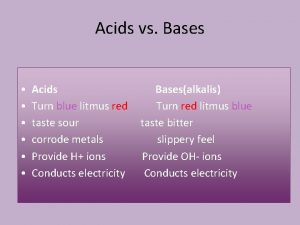
Ammonia Damp, red litmus paper turns blue. Chlorine Damp blue litmus paper. first turns red showing an acidic nature and is then bleached white.

Oxygen Hydrogen This burns with a squeaky pop when a lighted splint is used A glowing splint, relights

Test for Carbon Dioxide CO 2 bubbled through limewater: Ca(OH)2 1) turns the water cloudy 2) returns to clear 1) 2) Ca(OH)2(aq) + CO 2(g) ⇒ Ca. CO 3(s) + H 2 O(l) Ca(HCO 3)2(aq)

Test for Sulfur Dioxide Drop potassium manganate(VII) onto a piece of filter paper The purple colour will turn red then orange and finally it will be bleached

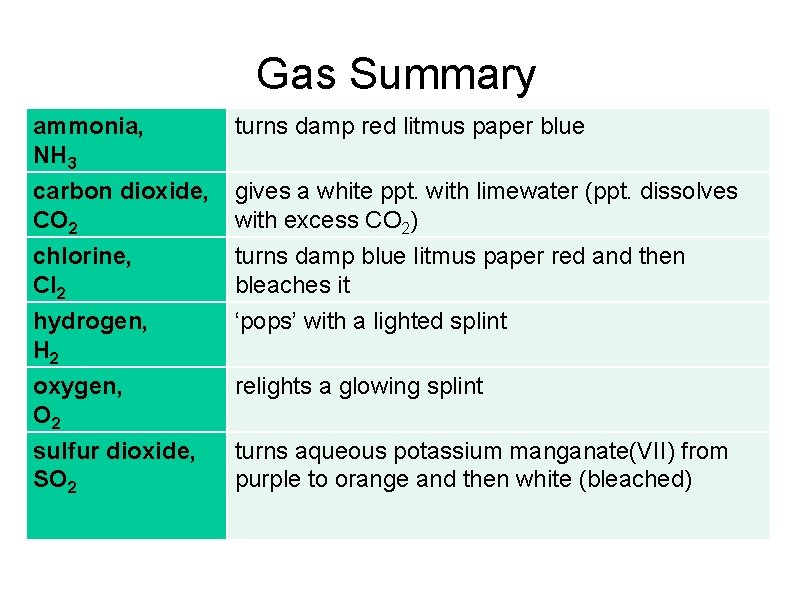
Gas Summary ammonia, NH 3 carbon dioxide, CO 2 turns damp red litmus paper blue chlorine, Cl 2 hydrogen, H 2 turns damp blue litmus paper red and then bleaches it ‘pops’ with a lighted splint oxygen, O 2 sulfur dioxide, SO 2 relights a glowing splint gives a white ppt. with limewater (ppt. dissolves with excess CO 2) turns aqueous potassium manganate(VII) from purple to orange and then white (bleached)

Testing for Anions

Test for carbonates Add a dilute acid. Fizzing shows a positive result. Bubble carbon dioxide gas through lime water Ca(OH)2 (aq) + CO 2 (g) Ca. CO 3 (s)

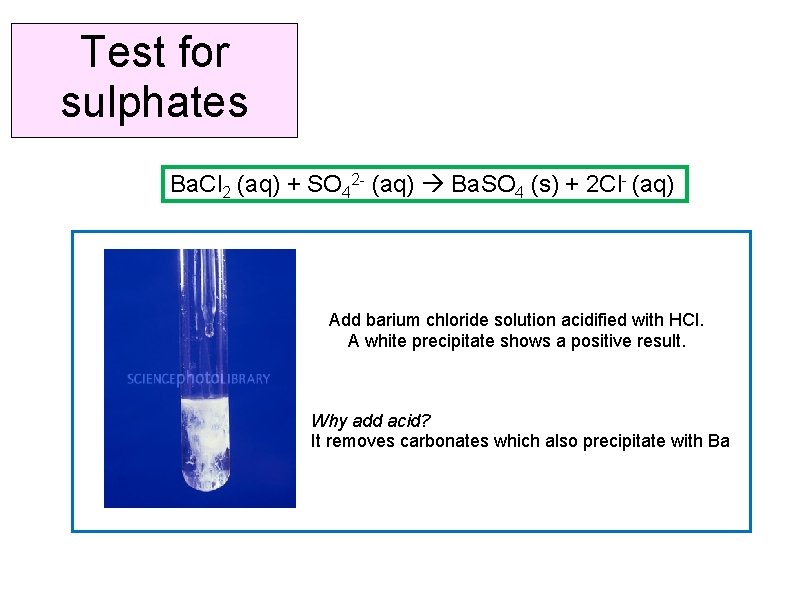
Test for sulphates Ba. Cl 2 (aq) + SO 42 - (aq) Ba. SO 4 (s) + 2 Cl- (aq) Add barium chloride solution acidified with HCl. A white precipitate shows a positive result. Why add acid? It removes carbonates which also precipitate with Ba

Test for sulphites 1. Add acid to the sulphite 2. Warm Gently 3. Test the gas produced with KMn. O 4 on filter paper 4. The purple colour will turn red then orange and finally it will be bleached

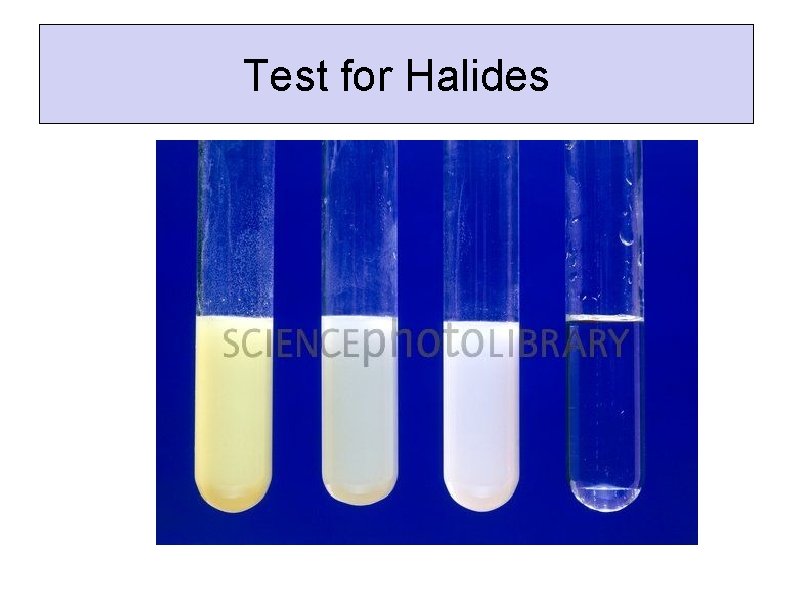
Test for Halides

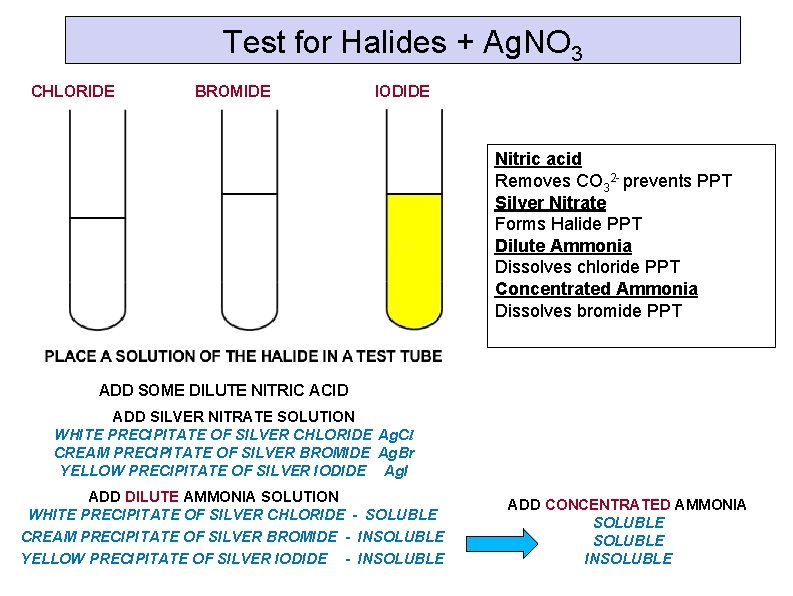
Test for Halides + Ag. NO 3 CHLORIDE BROMIDE IODIDE Nitric acid Removes CO 32 - prevents PPT Silver Nitrate Forms Halide PPT Dilute Ammonia Dissolves chloride PPT Concentrated Ammonia Dissolves bromide PPT ADD SOME DILUTE NITRIC ACID ADD SILVER NITRATE SOLUTION WHITE PRECIPITATE OF SILVER CHLORIDE Ag. Cl CREAM PRECIPITATE OF SILVER BROMIDE Ag. Br YELLOW PRECIPITATE OF SILVER IODIDE Ag. I ADD DILUTE AMMONIA SOLUTION WHITE PRECIPITATE OF SILVER CHLORIDE - SOLUBLE CREAM PRECIPITATE OF SILVER BROMIDE - INSOLUBLE YELLOW PRECIPITATE OF SILVER IODIDE - INSOLUBLE ADD CONCENTRATED AMMONIA SOLUBLE INSOLUBLE

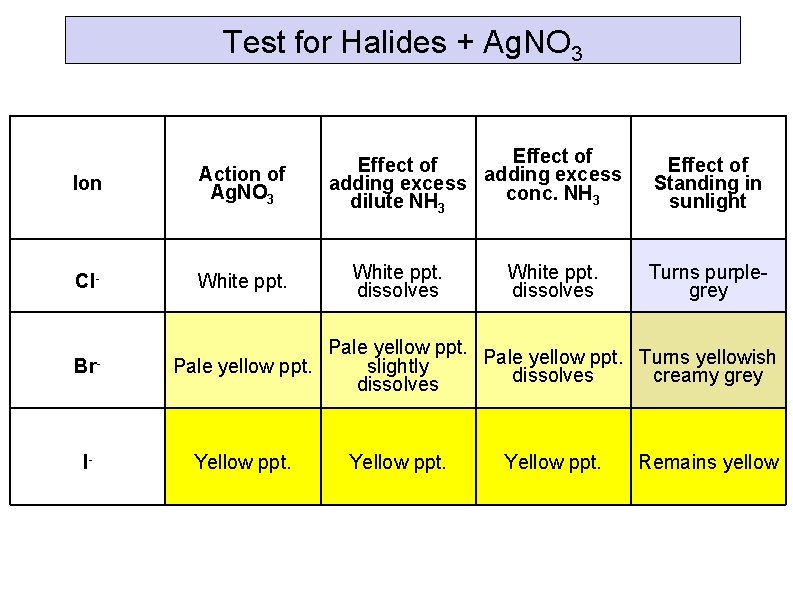
Test for Halides + Ag. NO 3 Ion Action of Ag. NO 3 Cl- White ppt. Br- I- Effect of excess adding conc. NH 3 dilute NH 3 White ppt. dissolves Effect of Standing in sunlight Turns purplegrey Pale yellow ppt. Turns yellowish Pale yellow ppt. slightly dissolves creamy grey dissolves Yellow ppt. Remains yellow

Test for Nitrates Test for the nitrate ion • Add a few drops of dilute sodium hydroxide • Add some aluminium powder. • Heat • This produces ammonium ions which break down to form ammonia. • Test with damp red litmus paper blue Ca(NO 3)2 (aq) + Na. OH (aq) + Al (s) NH 3 (g) + others that you don't need to know

What is the test?

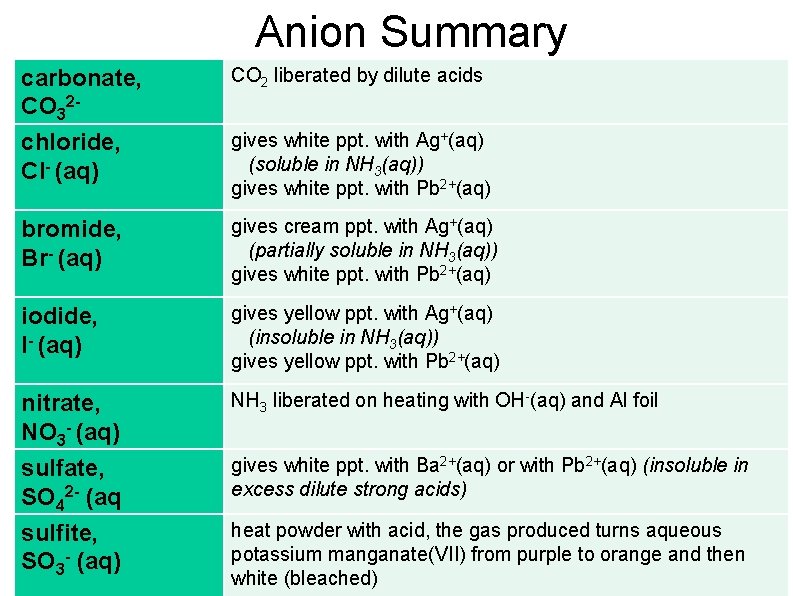
Anion Summary carbonate, CO 32 chloride, Cl- (aq) CO 2 liberated by dilute acids bromide, Br- (aq) gives cream ppt. with Ag+(aq) (partially soluble in NH 3(aq)) gives white ppt. with Pb 2+(aq) iodide, I- (aq) gives yellow ppt. with Ag+(aq) (insoluble in NH 3(aq)) gives yellow ppt. with Pb 2+(aq) nitrate, NO 3 - (aq) NH 3 liberated on heating with OH-(aq) and Al foil sulfate, SO 42 - (aq gives white ppt. with Ba 2+(aq) or with Pb 2+(aq) (insoluble in excess dilute strong acids) sulfite, SO 3 - (aq) heat powder with acid, the gas produced turns aqueous potassium manganate(VII) from purple to orange and then white (bleached) gives white ppt. with Ag+(aq) (soluble in NH 3(aq)) gives white ppt. with Pb 2+(aq)


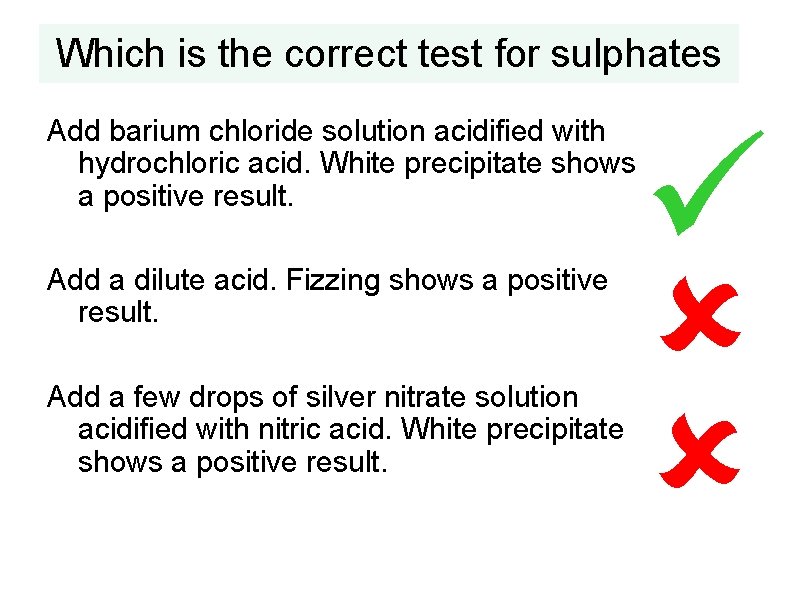
Which is the correct test for sulphates Add barium chloride solution acidified with hydrochloric acid. White precipitate shows a positive result. Add a dilute acid. Fizzing shows a positive result. Add a few drops of silver nitrate solution acidified with nitric acid. White precipitate shows a positive result.

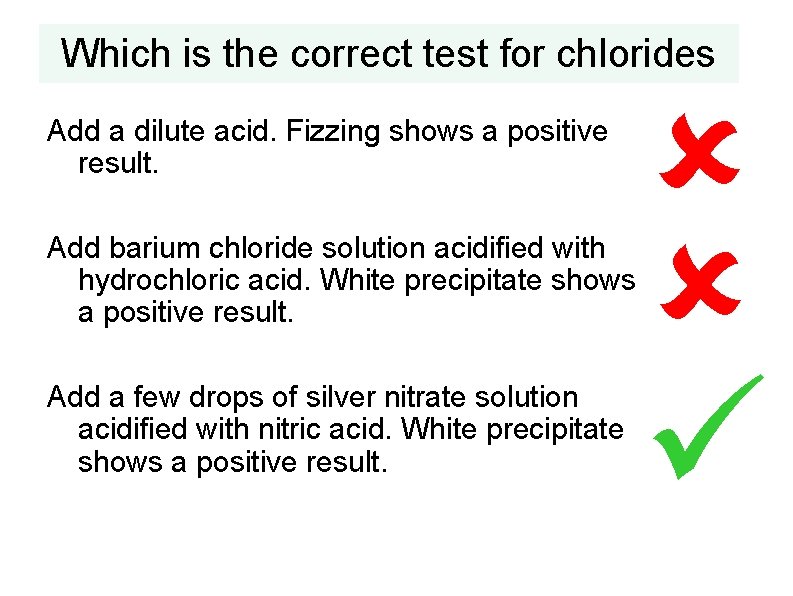
Which is the correct test for chlorides Add a dilute acid. Fizzing shows a positive result. Add barium chloride solution acidified with hydrochloric acid. White precipitate shows a positive result. Add a few drops of silver nitrate solution acidified with nitric acid. White precipitate shows a positive result.

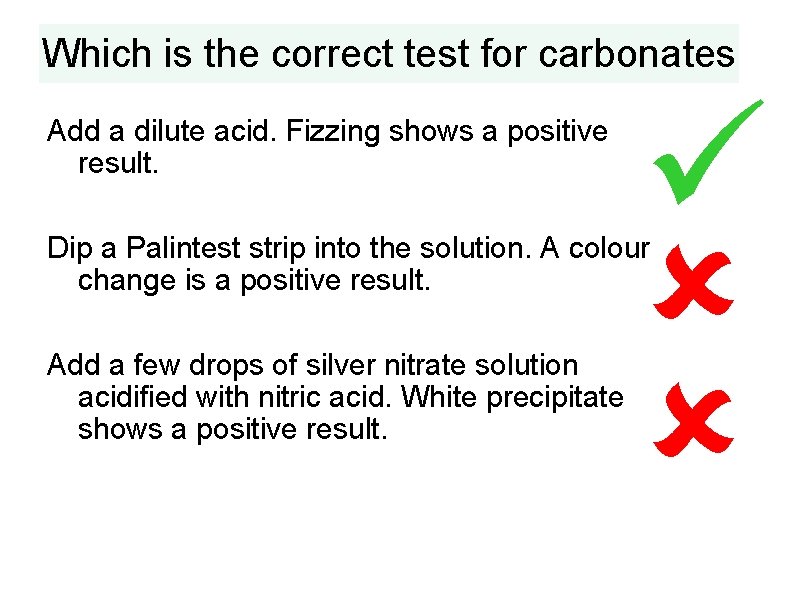
Which is the correct test for carbonates Add a dilute acid. Fizzing shows a positive result. Dip a Palintest strip into the solution. A colour change is a positive result. Add a few drops of silver nitrate solution acidified with nitric acid. White precipitate shows a positive result.

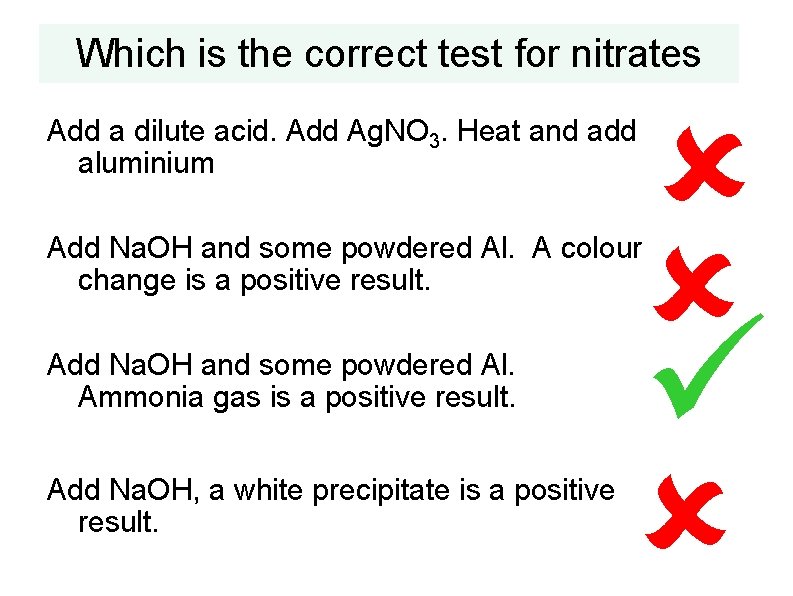
Which is the correct test for nitrates Add a dilute acid. Add Ag. NO 3. Heat and add aluminium Add Na. OH and some powdered Al. A colour change is a positive result. Add Na. OH and some powdered Al. Ammonia gas is a positive result. Add Na. OH, a white precipitate is a positive result.


What Am I? I'm form a white ppt. With Ag+ ions My ppt. dissolves in dilute NH 3 Cl

What Am I? I fizz with dilute acids CO 3 2 -

What Am I? I'm form a yellow ppt. With Ag+ ions My ppt. does not dissolve in dilute NH 3 My ppt. does not dissolve in conc. NH 3 I

What Am I? I'm form ammonia gas when heated with powdered Al and Na. OH NO 3 -

What Am I? I'm form a ppt. With Ag+ ions as shown My ppt. changes colour on exposure to light as shown Br

What Am I? The addition of dilute nitric acid and then barium chloride forms a white ppt. SO 4 2 -

What Am I? I'm form a ppt. With Ag+ ions as shown My ppt. changes colour on exposure to light as shown Cl

Flame Tests

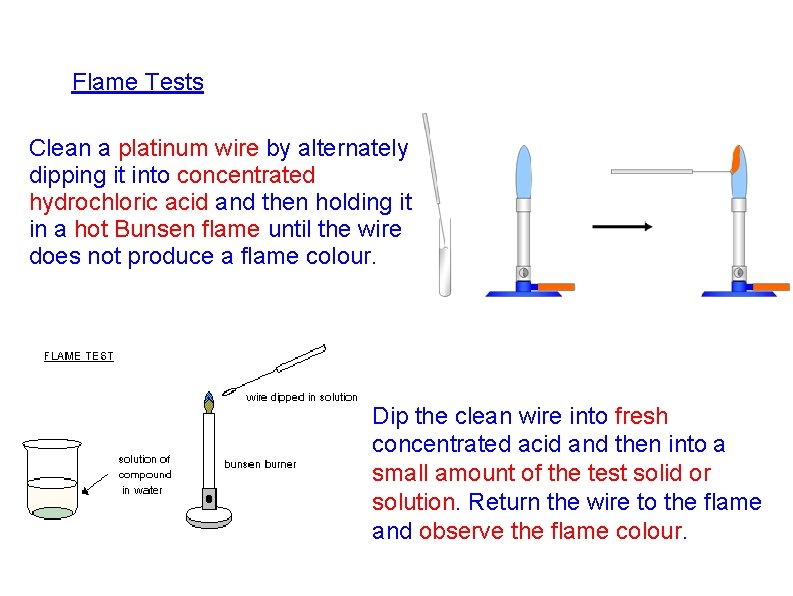
Flame Tests Clean a platinum wire by alternately dipping it into concentrated hydrochloric acid and then holding it in a hot Bunsen flame until the wire does not produce a flame colour. Dip the clean wire into fresh concentrated acid and then into a small amount of the test solid or solution. Return the wire to the flame and observe the flame colour.

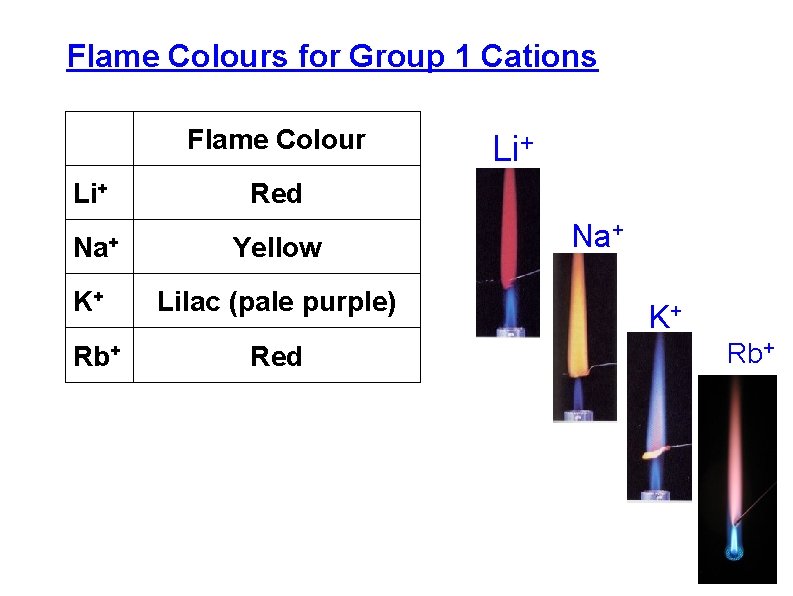
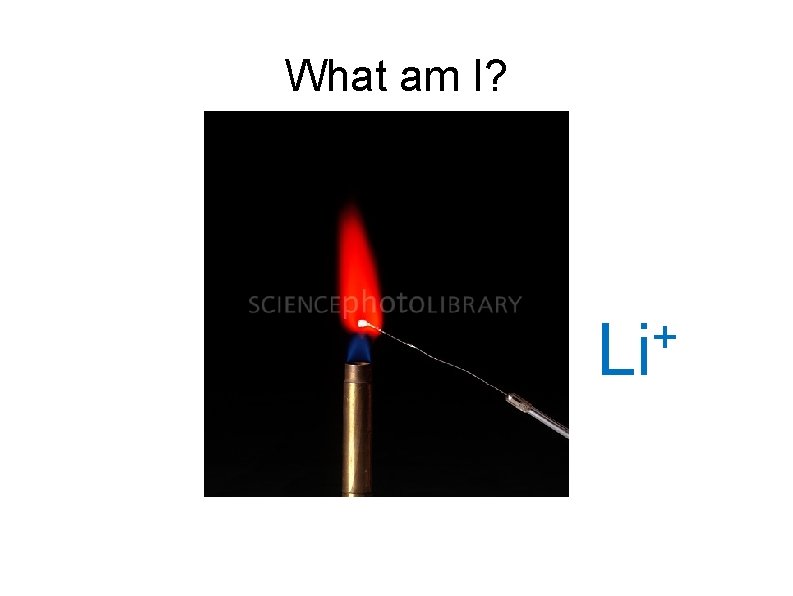
Flame Colours for Group 1 Cations Flame Colour Li+ Red Na+ Yellow K+ Lilac (pale purple) Rb+ Li+ Red Na+ K+ Rb+

Flame Colour for Copper Flame Colour Cu 2+ Green-Blue Cu 2+

What am I? + Na 2+ Ca

What am I? 2+ Cu

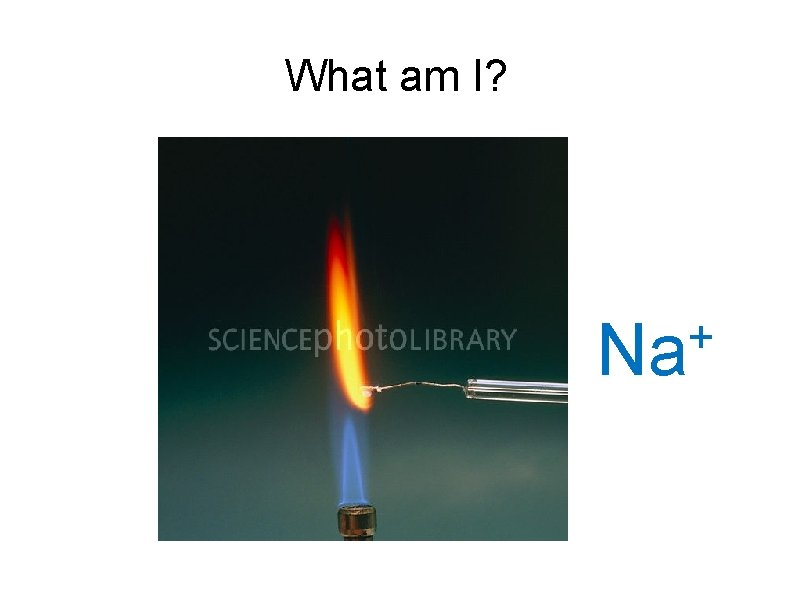
What am I? + Na

What am I? + Li 2+ Sr

What am I? + K

What are we? 2+ Cu & + Na

What am I?

• A flame test gave • the colour below • Testing with acid and then • Lime Water gave the result • below • Sodium Carbonate • Na 2 CO 3

• A flame test gave • the colour below • Testing with silver nitrate only • gave the result below, the ppt. • dissolved in dilute NH 3 • Sodium Chloride • Na. Cl

• A flame test gave • the colour below • Testing with silver nitrate only • gave the result below, the ppt. • did not dissolve in NH 3 • Potassium Iodide • KI

• What am I? • Red flame • during a flame test • Lithium Bromide • Li. Br • Test with silver nitrate • → cream ppt. and the • ppt. should dissolve in • conc. NH 3

• A flame test gave • the colour below • Testing with Na. OH + Al and heat • produced NH 3 gas • Potassium Nitrate • KNO 3

• What tests would you do to prove you had: • copper sulfate (Cu. SO 4) • and what would the results be? • Cu 2+ • Blue-green flame • during a flame test • A white ppt. on adding • barium chloride (Ba. Cl 2)

• NOW! • In pairs write down 2 problems of your own and then ask other students to solve your question. • e. g. What compound is this? A red flame was produced during a flame test and on addition of acid a gas was evolved which turned lime water cloudy.

Aqueous Cations

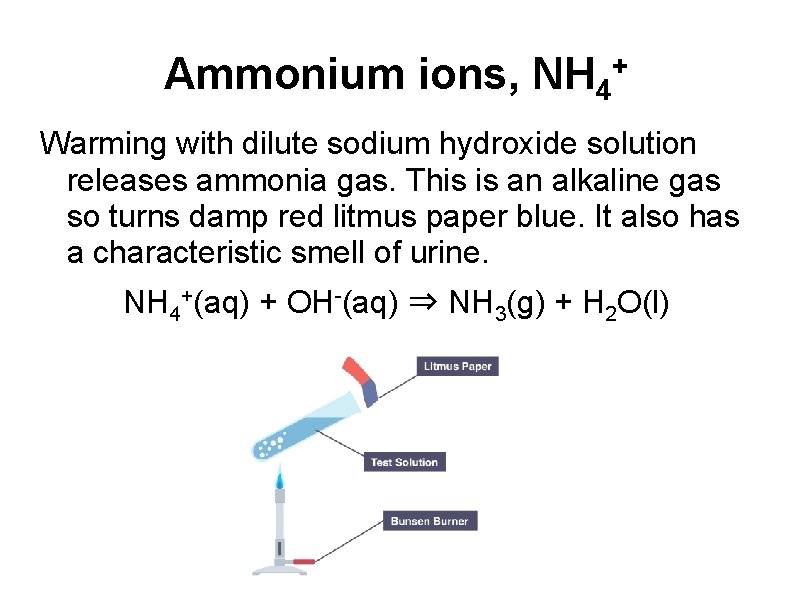
Ammonium ions, NH 4 + Warming with dilute sodium hydroxide solution releases ammonia gas. This is an alkaline gas so turns damp red litmus paper blue. It also has a characteristic smell of urine. NH 4+(aq) + OH-(aq) ⇒ NH 3(g) + H 2 O(l)

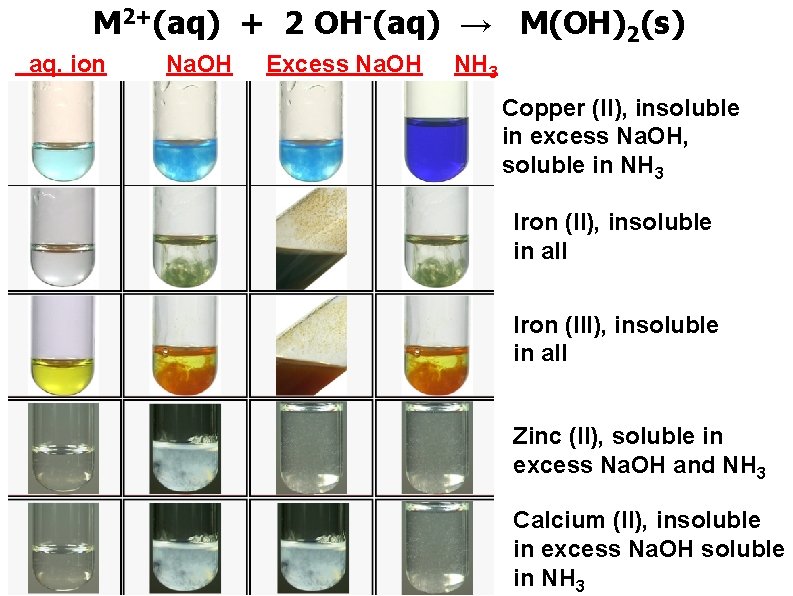
M 2+(aq) + 2 OH-(aq) → M(OH)2(s) aq. ion Na. OH Excess Na. OH NH 3 Copper (II), insoluble in excess Na. OH, soluble in NH 3 Iron (II), insoluble in all Iron (III), insoluble in all Zinc (II), soluble in excess Na. OH and NH 3 Calcium (II), insoluble in excess Na. OH soluble in NH 3

M 3+(aq) + 3 OH-(aq) → M(OH)3(s) aq. ion Na. OH Excess Na. OH NH 3 Chromium (III), soluble in excess Na. OH, insoluble in NH 3 Alumunium (III), soluble in excess Na. OH, insoluble in NH 3

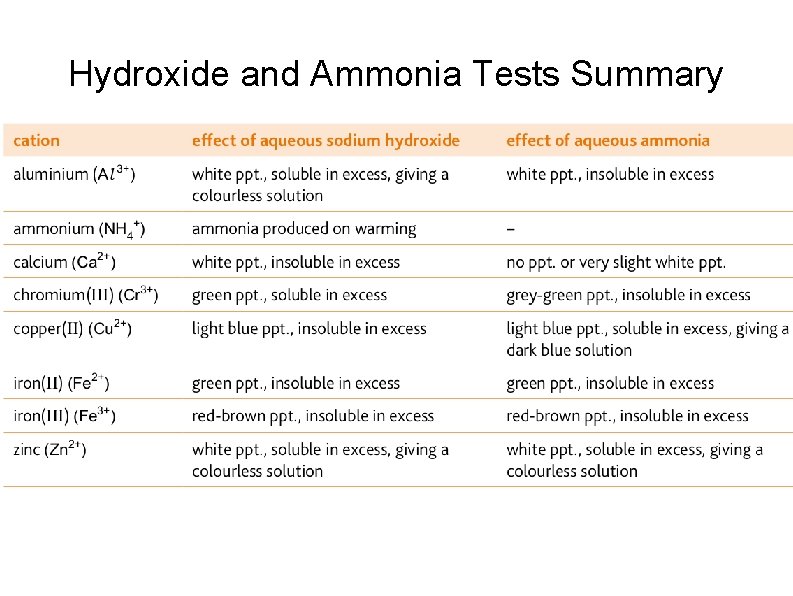
Hydroxide and Ammonia Tests Summary



Colour of transition metal ions in salts Give the colour 1) Iron (II) Pale green 2) Copper (II) or Chromium (III) Green 3) Chromate (VI) Yellow Colour of transition metal ions in dilute solution Give the colour or ion responsible for the colour 4) Iron (III) brown/yellow 5) Zinc (II) colourless 6) Blue copper (II)

Flame Tests Give the metal ion 7) Red lithium or strontium 8) Potassium lilac Common Gases Give the gas responsible or the test for that gas 9) Brown gas nitrogen dioxide or bromine 10) Iodine purple vapour 11) Water turns blue cobalt chloride paper pink

Colour of transition metal ions in salts Give the colour 1) manganate (VII) PURPLE 2) dichromate (VI) ORANGE 3) manganese (II) PINK Colour of transition metal ions in dilute solution Give the colour or ion responsible for the colour 4) manganese (II) PALE PINK 5) manganate (VII) PURPLE 6) yellow CHROMATE (VI)

Flame Tests Give the metal ion 7) yellow-red CALCIUM 8) pale-green BARIUM Common Gases Give the gas responsible or the test for that gas 9) pale-green gas which bleaches moist litmus paper 10) hydrogen chloride 11) oxygen CHLORINE STEAMY FUMES ON EXPOSURE TO MOIST AIR, ACIDIC AND FORMS WHITE SMOKE WITH AMMONIA COLOURLESS GAS WHICH RELIGHTS A GLOWING SPLINT
 Damp blue litmus paper in chlorine gas
Damp blue litmus paper in chlorine gas Blue litmus paper turns red
Blue litmus paper turns red 7))chlorine dissolved in water
7))chlorine dissolved in water What colour would blue litmus paper be in orange juice
What colour would blue litmus paper be in orange juice Stormy clot reaction
Stormy clot reaction Litmus milk broth
Litmus milk broth Sifat asid
Sifat asid What is a base
What is a base Litmus is extracted from
Litmus is extracted from Oxidase negative
Oxidase negative Acids have a taste and they turn litmus to
Acids have a taste and they turn litmus to Energy
Energy Acids have a taste and they turn litmus to
Acids have a taste and they turn litmus to A company's environmental sustainability strategy concerns
A company's environmental sustainability strategy concerns Litmus bulletproof buttons
Litmus bulletproof buttons Damp sweeping
Damp sweeping Damp spoon theory
Damp spoon theory Pressing garments
Pressing garments Damp scrl
Damp scrl Damp open systeem behang
Damp open systeem behang Damp folie
Damp folie Urea cycle definition
Urea cycle definition Fsfffs
Fsfffs Trigonal pyramidal with lone pair
Trigonal pyramidal with lone pair Gdd
Gdd Liquid ammonia as non aqueous solvent
Liquid ammonia as non aqueous solvent Haber process chart
Haber process chart Cold storage
Cold storage Definition of buffering capacity
Definition of buffering capacity Ammonia mo diagram
Ammonia mo diagram Mangalore chemicals and fertilizers parent organizations
Mangalore chemicals and fertilizers parent organizations Ostwald process
Ostwald process Can fused calcium chloride dry ammonia
Can fused calcium chloride dry ammonia Threshold limit value of ammonia
Threshold limit value of ammonia Ammonia electrolyzer
Ammonia electrolyzer Ammonia
Ammonia Ammonia
Ammonia Alcohol + ammonia
Alcohol + ammonia Perma pure ammonia scrubber
Perma pure ammonia scrubber Aqueous ammonia
Aqueous ammonia Ammonia awareness safety policy
Ammonia awareness safety policy Unscramble ammonia
Unscramble ammonia Flavouring agents used in aromatic spirit of ammonia
Flavouring agents used in aromatic spirit of ammonia Ammónia képlete
Ammónia képlete Many freshwater invertebrates eliminate ammonia by
Many freshwater invertebrates eliminate ammonia by English general paper paper 2 comprehension
English general paper paper 2 comprehension Aice general paper 1
Aice general paper 1 Domain closure in software testing
Domain closure in software testing Logic based testing
Logic based testing Data flow testing strategies in software testing
Data flow testing strategies in software testing Positive vs negative testing
Positive vs negative testing Cs 3250
Cs 3250 Globalization testing in software testing
Globalization testing in software testing Neighborhood integration testing
Neighborhood integration testing Cause effect graphing technique
Cause effect graphing technique Control structure testing in software testing
Control structure testing in software testing Decision table testing in software testing
Decision table testing in software testing Decision table for triangle problem
Decision table for triangle problem Apa itu black box testing
Apa itu black box testing