UREA CYCLE MECHANISM OF AMMONIA TOXICITY Ammonia is







- Slides: 30

UREA CYCLE.


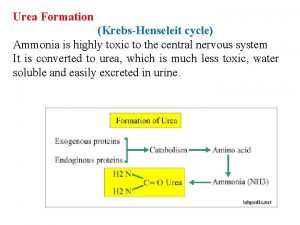
MECHANISM OF AMMONIA TOXICITY Ammonia is a very toxic substance, and usually excreted in the form of urea. Metabolic disorders that arise from abnormal function of enzymes Of urea synthesis (urea cycle) are fatal and cause coma “due to ATP depletion”, specially when ammonia concentration is high. High concentration of ammonia sequesters α ketoglutarate in the form of Glutamate, leading to: depletion of TCA cycle intermediates and reducing ATP production. 3

TRANSPORT OF AMMONIA Ammonia transporter (glutamine) Ammonia generated from N 2 is assimilated into low molecular weight metabolites such as glutamate or glutamine 50% of circulating amino acid molecules are glutamine. The amide group of glutamine is a nitrogen donor for several classes of Molecules including: purine bases, and the amino group of cystine. Glutamate and ammonia are the substrates for glutamine synthetase. Removal of the amide group is catalyzed by glutaminase 4

TRANSPORT OF NITROGEN TO LIVER AND KIDNEY Muscle protein responds to conditions such as: starvation, trauma, Burns, and septicemia by undergoing massive degradation. - The most percentage of body protein is in skeletal muscle. - Under conditions of energy need, this protein is degraded and amino groups from amino acids are transferred to produce glutamine and alanine. -Finally, these amino acids “glutamine and alanine” are transported to Liver and kidney to produce urea and ammonia. 5

6

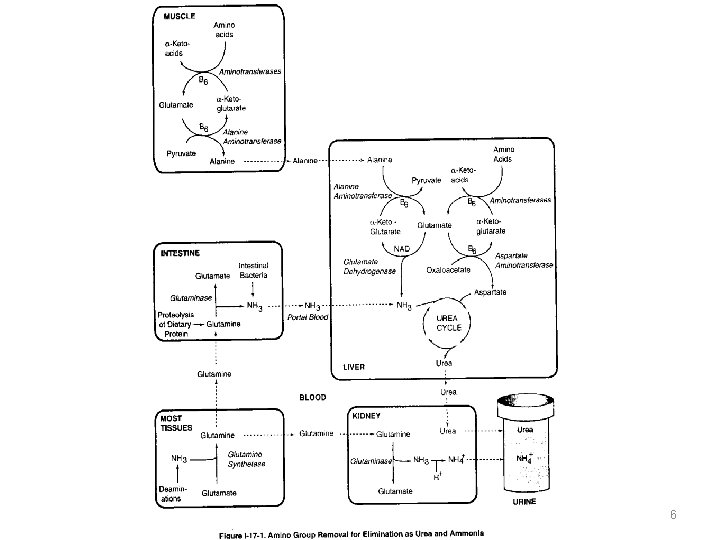
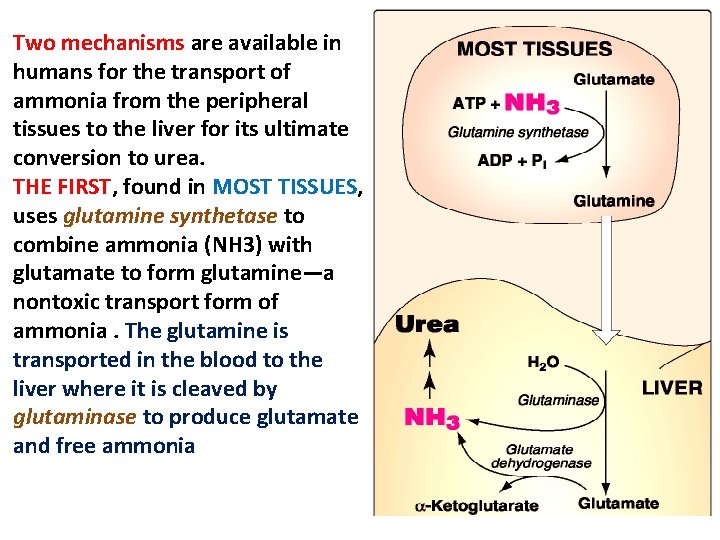
Two mechanisms are available in humans for the transport of ammonia from the peripheral tissues to the liver for its ultimate conversion to urea. THE FIRST, found in MOST TISSUES, uses glutamine synthetase to combine ammonia (NH 3) with glutamate to form glutamine—a nontoxic transport form of ammonia. The glutamine is transported in the blood to the liver where it is cleaved by glutaminase to produce glutamate and free ammonia

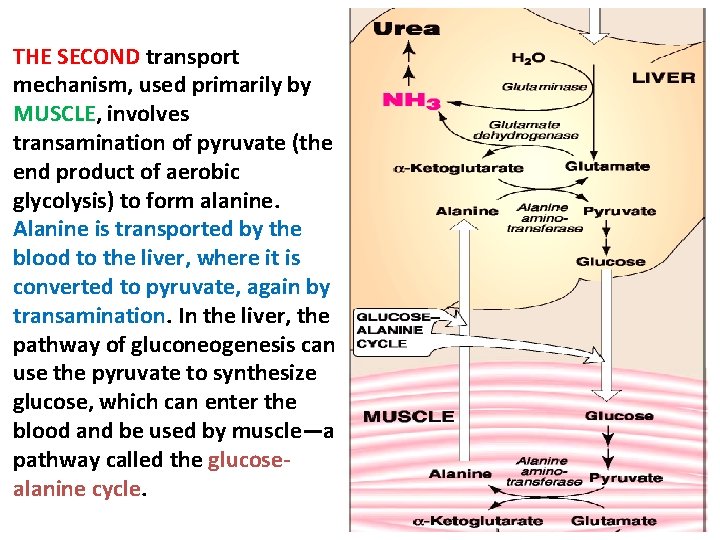
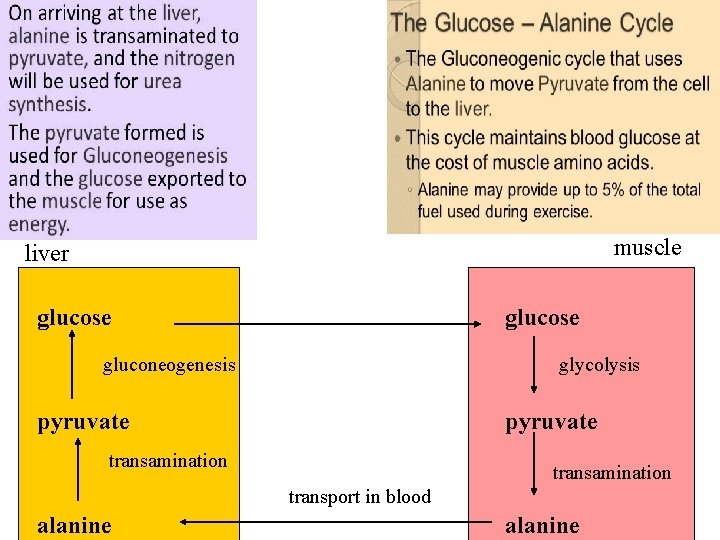
THE SECOND transport mechanism, used primarily by MUSCLE, involves transamination of pyruvate (the end product of aerobic glycolysis) to form alanine. Alanine is transported by the blood to the liver, where it is converted to pyruvate, again by transamination. In the liver, the pathway of gluconeogenesis can use the pyruvate to synthesize glucose, which can enter the blood and be used by muscle—a pathway called the glucosealanine cycle.

muscle liver glucose gluconeogenesis glycolysis pyruvate transamination transport in blood alanine 9

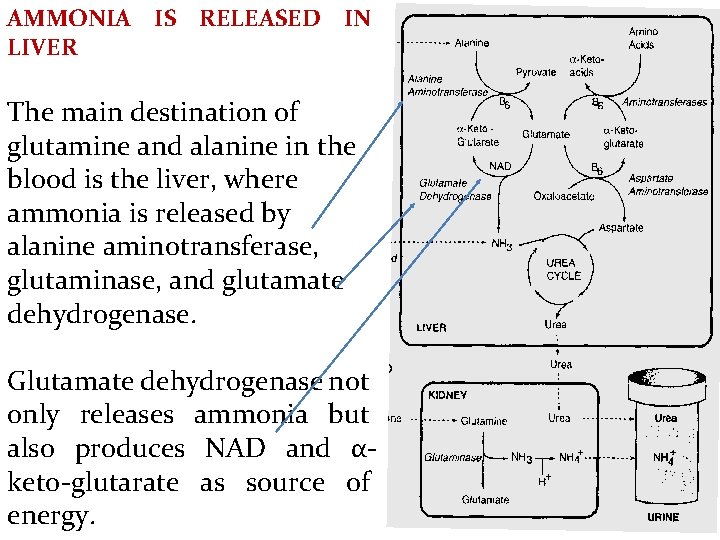
AMMONIA IS RELEASED IN LIVER The main destination of glutamine and alanine in the blood is the liver, where ammonia is released by alanine aminotransferase, glutaminase, and glutamate dehydrogenase. Glutamate dehydrogenase not only releases ammonia but also produces NAD and αketo-glutarate as source of energy.

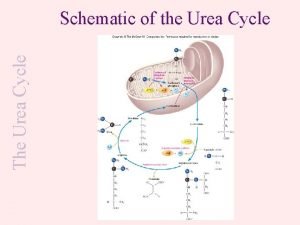
UREA CYCLE The urea cycle is the major mechanism for utilization and excretion of ammonia (excretion of ammonia in form of urea in the kidneys). In urea cycle: urea will be produced. Since humans cannot metabolize Urea, it is transported to the kidneys for filtration and excretion. 11

REACTIONS OF THE UREA CYCLE

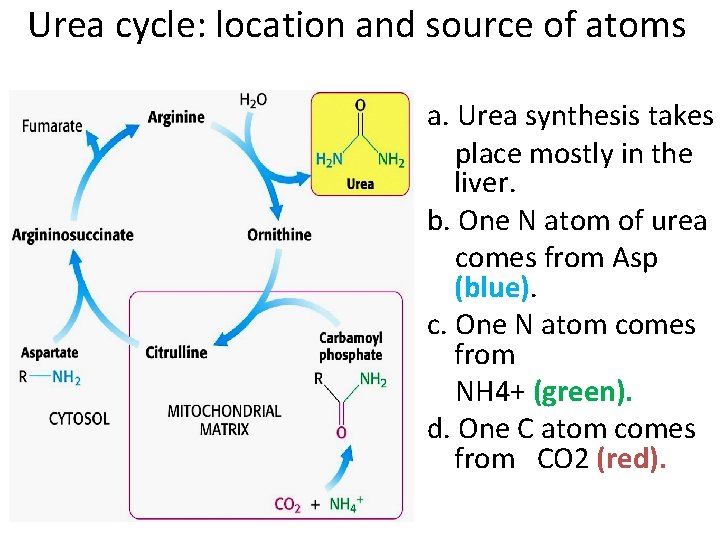
Urea cycle: location and source of atoms a. Urea synthesis takes place mostly in the liver. b. One N atom of urea comes from Asp (blue). c. One N atom comes from NH 4+ (green). d. One C atom comes from CO 2 (red).

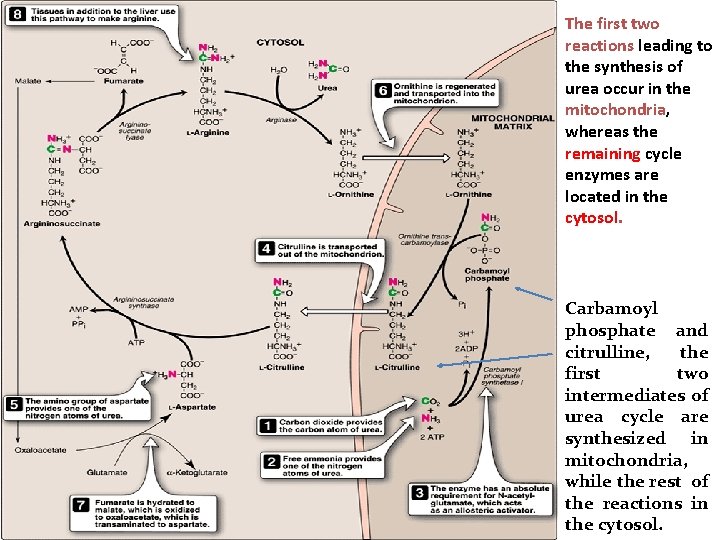
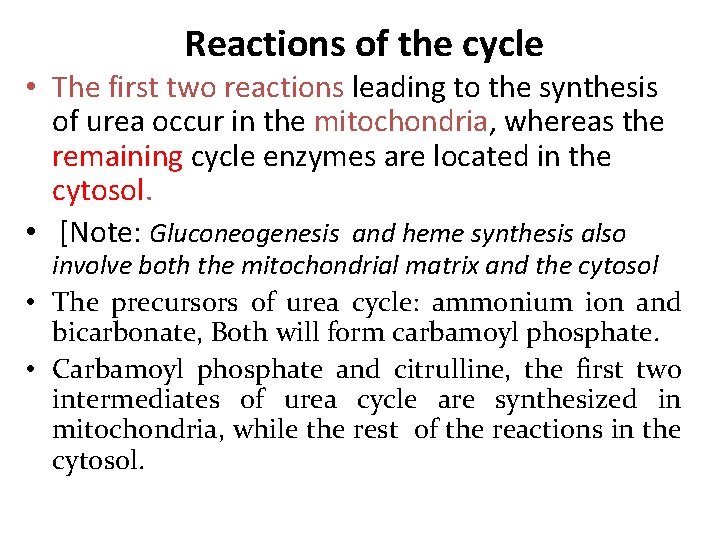
The first two reactions leading to the synthesis of urea occur in the mitochondria, whereas the remaining cycle enzymes are located in the cytosol. Carbamoyl phosphate and citrulline, the first two intermediates of urea cycle are synthesized in mitochondria, while the rest of the reactions in the cytosol.

Reactions of the cycle • The first two reactions leading to the synthesis of urea occur in the mitochondria, whereas the remaining cycle enzymes are located in the cytosol. • [Note: Gluconeogenesis and heme synthesis also involve both the mitochondrial matrix and the cytosol • The precursors of urea cycle: ammonium ion and bicarbonate, Both will form carbamoyl phosphate. • Carbamoyl phosphate and citrulline, the first two intermediates of urea cycle are synthesized in mitochondria, while the rest of the reactions in the cytosol.

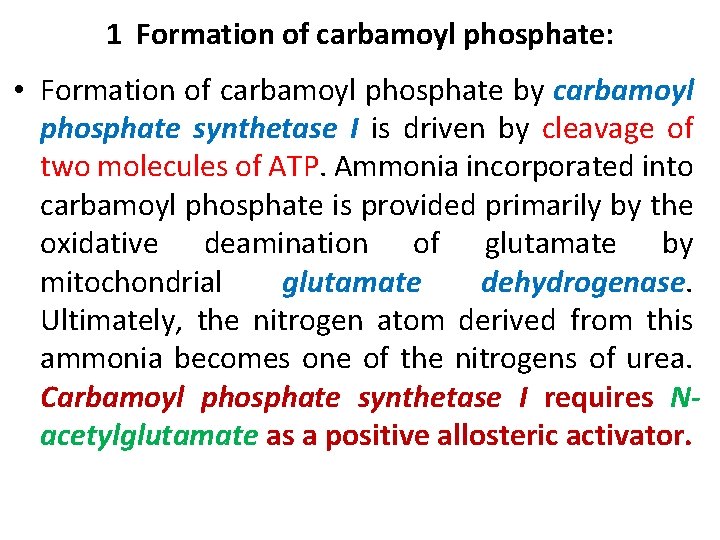
1 Formation of carbamoyl phosphate: • Formation of carbamoyl phosphate by carbamoyl phosphate synthetase I is driven by cleavage of two molecules of ATP. Ammonia incorporated into carbamoyl phosphate is provided primarily by the oxidative deamination of glutamate by mitochondrial glutamate dehydrogenase. Ultimately, the nitrogen atom derived from this ammonia becomes one of the nitrogens of urea. Carbamoyl phosphate synthetase I requires N acetylglutamate as a positive allosteric activator.

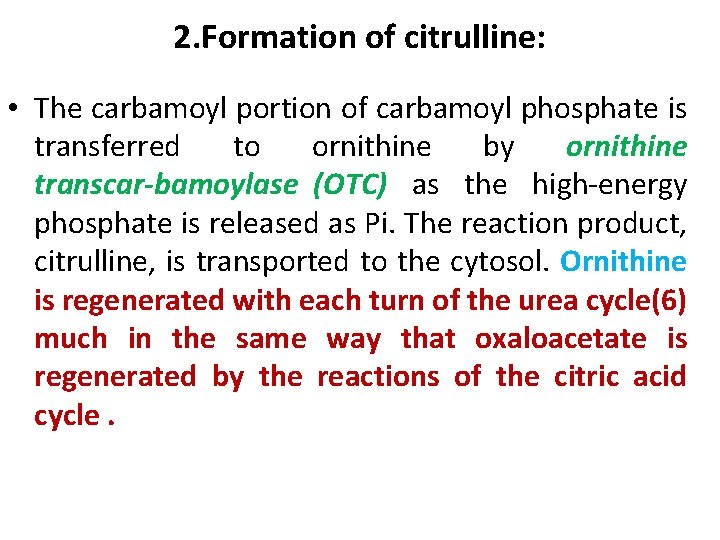
2. Formation of citrulline: • The carbamoyl portion of carbamoyl phosphate is transferred to ornithine by ornithine transcar bamoylase (OTC) as the high energy phosphate is released as Pi. The reaction product, citrulline, is transported to the cytosol. Ornithine is regenerated with each turn of the urea cycle(6) much in the same way that oxaloacetate is regenerated by the reactions of the citric acid cycle.

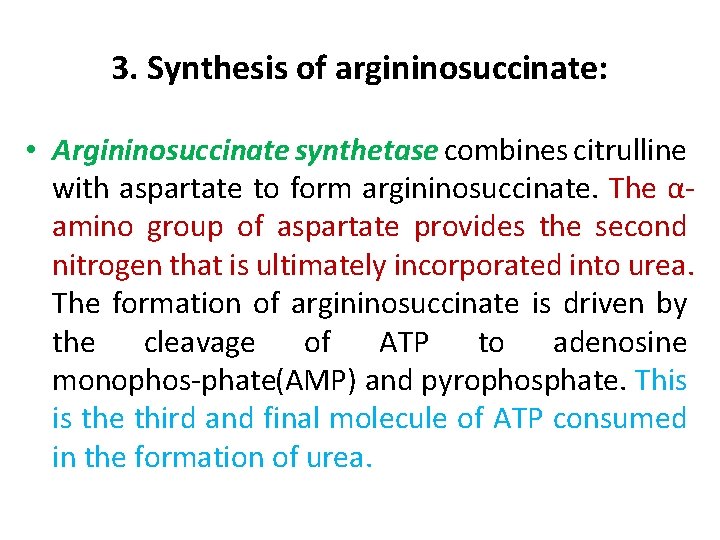
3. Synthesis of argininosuccinate: • Argininosuccinate synthetase combines citrulline with aspartate to form argininosuccinate. The α amino group of aspartate provides the second nitrogen that is ultimately incorporated into urea. The formation of argininosuccinate is driven by the cleavage of ATP to adenosine monophos phate(AMP) and pyrophosphate. This is the third and final molecule of ATP consumed in the formation of urea.

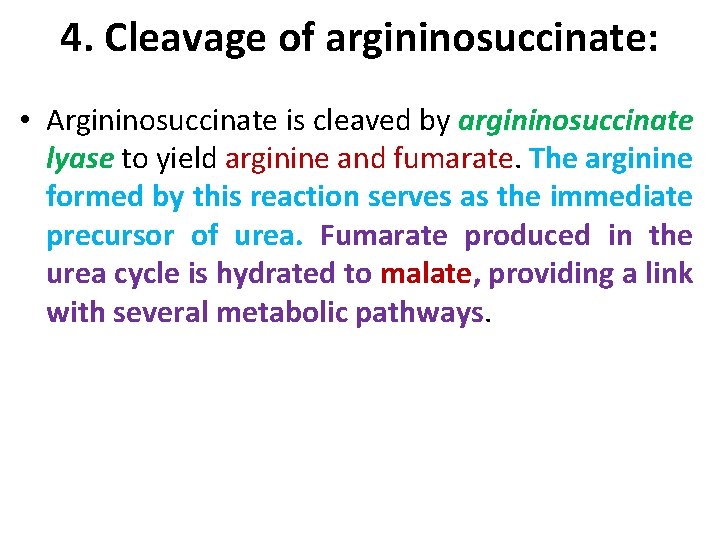
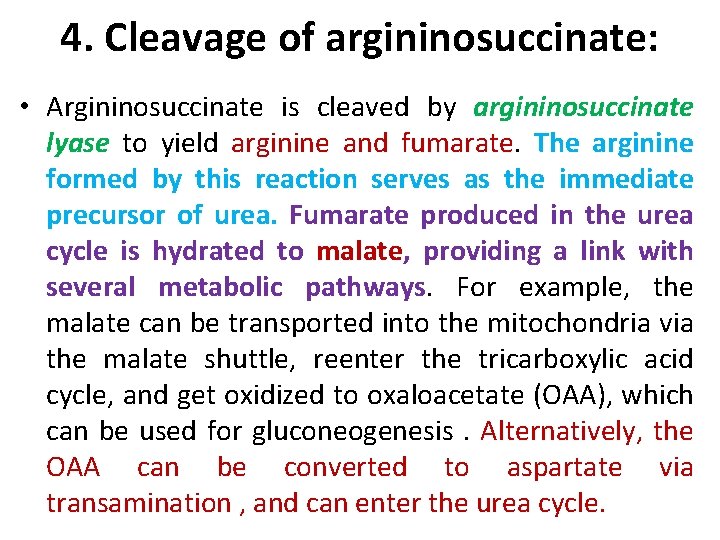
4. Cleavage of argininosuccinate: • Argininosuccinate is cleaved by argininosuccinate lyase to yield arginine and fumarate. The arginine formed by this reaction serves as the immediate precursor of urea. Fumarate produced in the urea cycle is hydrated to malate, providing a link with several metabolic pathways.

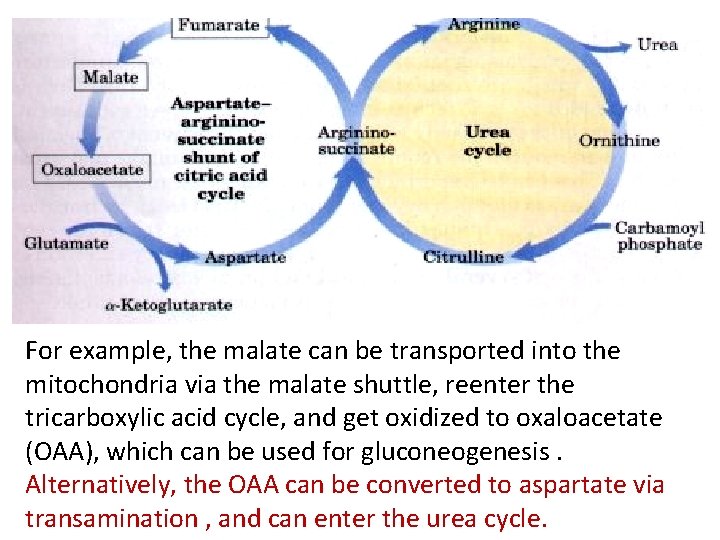
4. Cleavage of argininosuccinate: • Argininosuccinate is cleaved by argininosuccinate lyase to yield arginine and fumarate. The arginine formed by this reaction serves as the immediate precursor of urea. Fumarate produced in the urea cycle is hydrated to malate, providing a link with several metabolic pathways. For example, the malate can be transported into the mitochondria via the malate shuttle, reenter the tricarboxylic acid cycle, and get oxidized to oxaloacetate (OAA), which can be used for gluconeogenesis. Alternatively, the OAA can be converted to aspartate via transamination , and can enter the urea cycle.

Cleavage of arginine to ornithine and urea: • Arginase cleaves arginine to ornithine and urea, and occurs almost exclusively in the liver. Thus, whereas other tissues, such as the kidney, can synthesize arginine by these reactions, only the liver can cleave arginine and, thereby, synthesize urea.

For example, the malate can be transported into the mitochondria via the malate shuttle, reenter the tricarboxylic acid cycle, and get oxidized to oxaloacetate (OAA), which can be used for gluconeogenesis. Alternatively, the OAA can be converted to aspartate via transamination , and can enter the urea cycle.


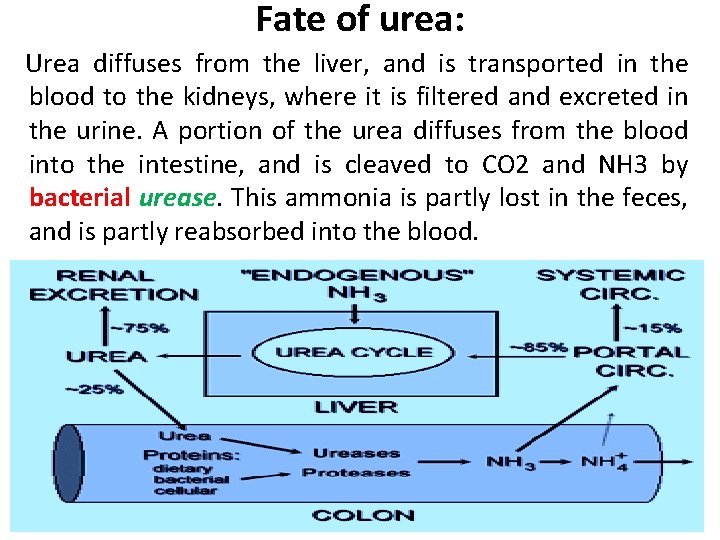
Fate of urea: Urea diffuses from the liver, and is transported in the blood to the kidneys, where it is filtered and excreted in the urine. A portion of the urea diffuses from the blood into the intestine, and is cleaved to CO 2 and NH 3 by bacterial urease. This ammonia is partly lost in the feces, and is partly reabsorbed into the blood.

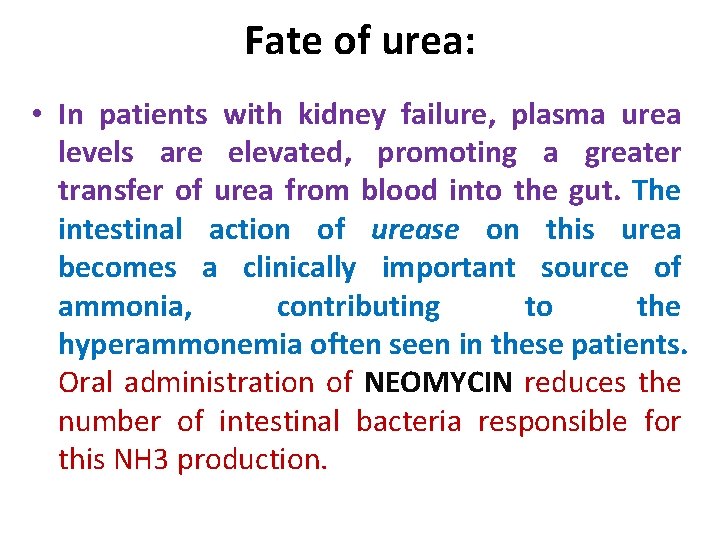
Fate of urea: • In patients with kidney failure, plasma urea levels are elevated, promoting a greater transfer of urea from blood into the gut. The intestinal action of urease on this urea becomes a clinically important source of ammonia, contributing to the hyperammonemia often seen in these patients. Oral administration of NEOMYCIN reduces the number of intestinal bacteria responsible for this NH 3 production.

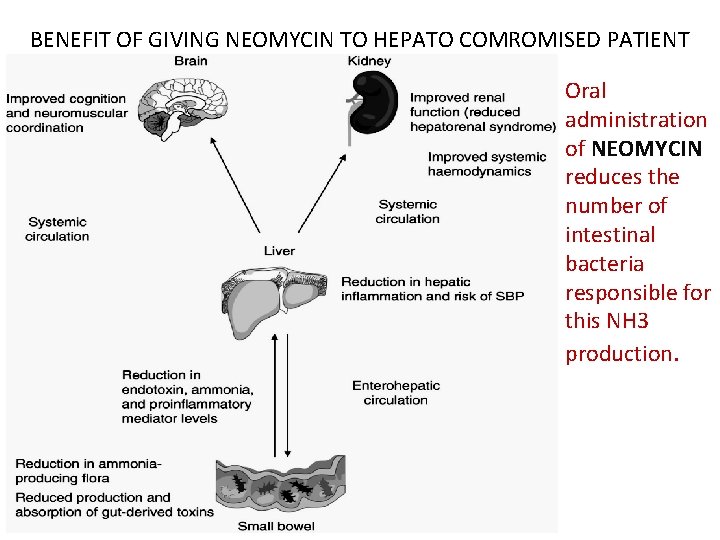
BENEFIT OF GIVING NEOMYCIN TO HEPATO COMROMISED PATIENT Oral administration of NEOMYCIN reduces the number of intestinal bacteria responsible for this NH 3 production.

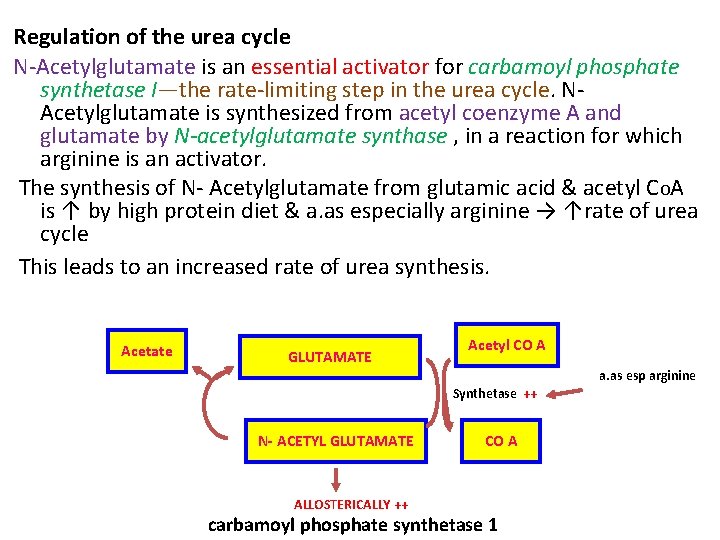
Regulation of the urea cycle N Acetylglutamate is an essential activator for carbamoyl phosphate synthetase I—the rate limiting step in the urea cycle. N Acetylglutamate is synthesized from acetyl coenzyme A and glutamate by N-acetylglutamate synthase , in a reaction for which arginine is an activator. The synthesis of N Acetylglutamate from glutamic acid & acetyl COA is ↑ by high protein diet & a. as especially arginine → ↑rate of urea cycle This leads to an increased rate of urea synthesis. Acetate GLUTAMATE Acetyl CO A a. as esp arginine Synthetase ++ N- ACETYL GLUTAMATE ALLOSTERICALLY ++ CO A carbamoyl phosphate synthetase 1

Overall stoichiometry of the urea cycle • Aspartate + NH 3 + CO 2 + 3 ATP + H 2 O ~ • urea + fumarate + 2 ADP + AMP + 2 Pi + Ppi • Three high energy phosphate bonds are consumed in the synthesis of each molecule of urea; therefore, the synthesis of urea is irreversible. [Note: The ATP is replenished by oxidative phosphorylation. ]

PNEUMONIC FOR UREA CYCLE

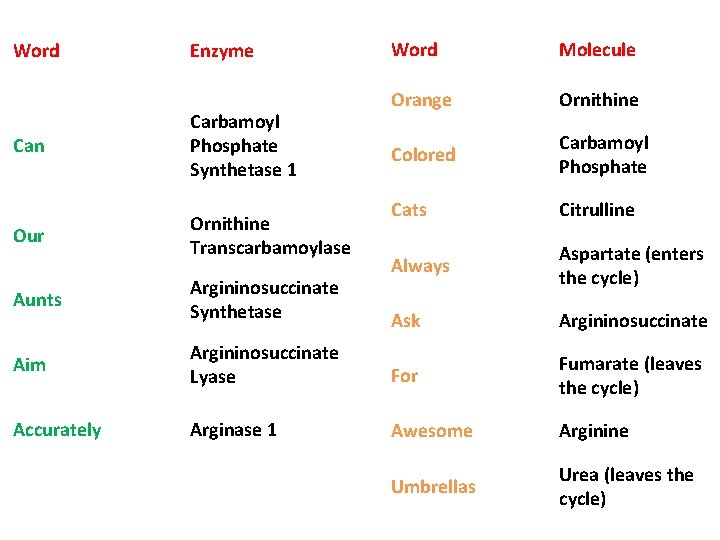
Word Enzyme Can Carbamoyl Phosphate Synthetase 1 Our Ornithine Transcarbamoylase Word Molecule Orange Ornithine Colored Carbamoyl Phosphate Cats Citrulline Always Aspartate (enters the cycle) Ask Argininosuccinate Aunts Argininosuccinate Synthetase Aim Argininosuccinate Lyase For Fumarate (leaves the cycle) Accurately Arginase 1 Awesome Arginine Umbrellas Urea (leaves the cycle)
 Urea cycle definition in biochemistry
Urea cycle definition in biochemistry Location of urea cycle
Location of urea cycle Reactions of urea cycle
Reactions of urea cycle Ornithine cycle
Ornithine cycle Urea cycle mnemonic
Urea cycle mnemonic Organic acidemia
Organic acidemia B6 toxicity symptoms
B6 toxicity symptoms Vitamin d toxicity
Vitamin d toxicity Definition of chronic toxicity
Definition of chronic toxicity Vitamin d toxicity
Vitamin d toxicity Severe preeclampsia criteria
Severe preeclampsia criteria Symptoms of potassium
Symptoms of potassium Oxygen poisoning
Oxygen poisoning Oxygen toxicity
Oxygen toxicity Organophosphate toxicity
Organophosphate toxicity Hepatic toxicity
Hepatic toxicity Lithium toxicity
Lithium toxicity Thyroid resistance syndrome
Thyroid resistance syndrome Ghs signal words
Ghs signal words Definition of chronic toxicity
Definition of chronic toxicity Toxicity symptoms
Toxicity symptoms Kaexylate
Kaexylate Anticholinergic syndrome
Anticholinergic syndrome Alcohol toxicity
Alcohol toxicity Roles of vitamin c
Roles of vitamin c Digoxin effects
Digoxin effects Role vitamin a
Role vitamin a Treatment of salicylate toxicity
Treatment of salicylate toxicity Water fluid
Water fluid Importance of toxicology
Importance of toxicology Thiamin
Thiamin