Acids Bases They are everywhere In your food

Acids & Bases They are everywhere. . In your food In your house EVEN IN YOU!!!!! 1 Dr Seemal Jelani 9/26/2020

What is an acid? § An acid is a solution that has an excess of H+ ions. It comes from the Latin word acidus that means "sharp" or "sour". § The more H + ions, the more acidic the solution. 2 Dr Seemal Jelani 9/26/2020
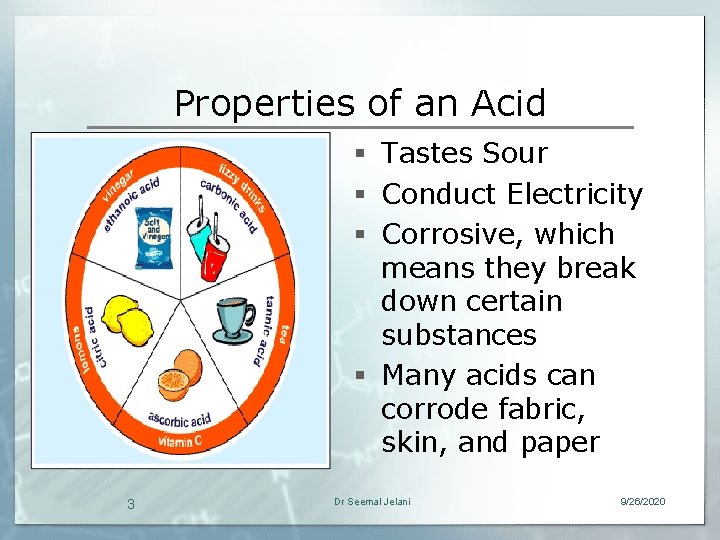
Properties of an Acid § Tastes Sour § Conduct Electricity § Corrosive, which means they break down certain substances § Many acids can corrode fabric, skin, and paper 3 Dr Seemal Jelani 9/26/2020

Introduction to Acids (con’t) § Lactic acid is also produced by bacteria in milk – this is why milk turns sour. § Acids occur naturally in many fruits. § Insects such as millipedes, scorpions, and ants use acids to deter predators. § A physician may use a solution of boric acid to rinse out your eyes, but a drop or two of many other acids would blind you. § Some acids add a tangy, sour flavour to foods and drinks, while others are deadly.

§ Some acids react strongly with metals § Turns blue litmus paper red 5 Dr Seemal Jelani 9/26/2020
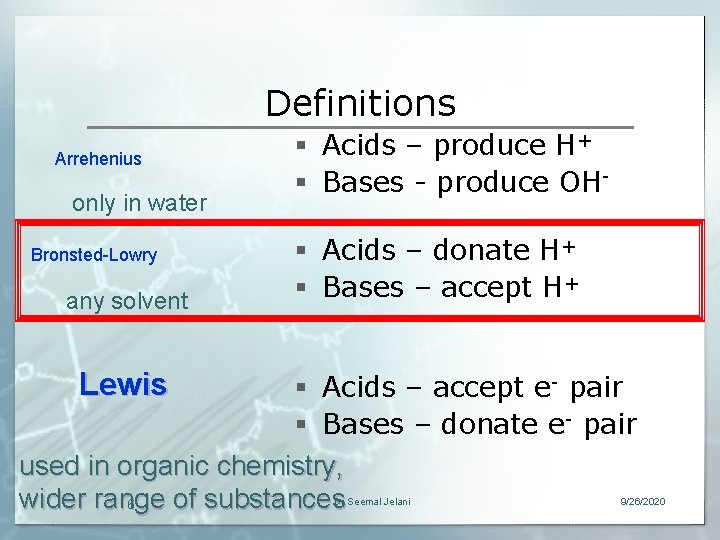
Definitions Arrehenius only in water Bronsted-Lowry any solvent § Acids – produce H+ § Bases - produce OH§ Acids – donate H+ § Bases – accept H+ Lewis § Acids – accept e- pair § Bases – donate e- pair used in organic chemistry, wider range of substances 6 Dr Seemal Jelani 9/26/2020
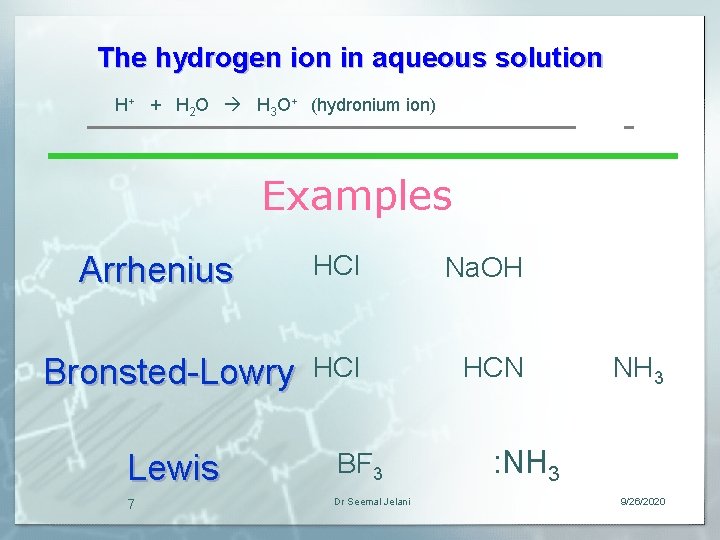
The hydrogen ion in aqueous solution H+ + H 2 O H 3 O+ (hydronium ion) Examples Arrhenius Bronsted-Lowry Lewis 7 HCl Na. OH HCl HCN BF 3 Dr Seemal Jelani NH 3 : NH 3 9/26/2020

Word Connect § The word “acid” comes from the Latin acidus, meaning “sour” § Think of the taste of fruits such as lemons or limes § These fruits contain citric acid § Vinegar, a common household product, contains acetic acid.

§ Chemists sometimes use the term “alkali” for a base that is soluble in water § This word shows the Arabic origin of chemistry § The earliest source of bases was the ash obtained by burning plants § Thus the Arabic word alkali means “ashes of a plant. ” 9 Dr Seemal Jelani 9/26/2020

Alkali salts are soluble hydroxides of alkali metals and alkaline earth metals, of which common examples are: § Sodium hydroxide – often called "caustic soda" § Potassium hydroxide – commonly called "caustic potash" § Lye – generic term for either of the previous two or even for a mixture § Salts of alkali § Reaction with water § What is an example of a strong alkali? (p. H 14) 10 Dr Seemal Jelani 9/26/2020

Strong acids and weak acids § High concentration of Hydrogen ions § Low concentration of Hydrogen ions § What is difference between base and alkali? § Bases are substances that react with acids and neutralize them. § They are usually metal oxides, metal hydroxides, metal carbonates or metal hydrogen carbonates. Many bases are insoluble - they do not dissolve in water. If a base does dissolve in water, we call it an alkali. 11 Dr Seemal Jelani 9/26/2020

§ What is an alkaline? § The “alkaline” in alkaline water refers to its p. H level. The p. H level is a number that measures how acidic or alkaline a substance is on a scale of 0 to 14 § Examples: acids, base, alkaline(p. H > 7. 0) 12 Dr Seemal Jelani 9/26/2020

§ An alkaline solution is a mixture of base solids dissolved in water. § The potential of hydrogen, also known as the p. H scale, measures the alkalinity or acidity level of a solution. § The scale ranges from zero to 14. The midpoint 7 represents a neutral p. H. § A neutral solution is neither an acid nor alkaline. p. H levels below 7 indicate an acidic solution, and numbers above 7 indicate an alkaline solution. § The p. H is a measurement of intensity, not Dr Seemal Jelani 9/26/2020 13 capacity

Is alkaline a base or acid? § The solution is neither acidic or basic. . Because the base "soaks up" hydrogen ions, the result is a solution with more hydroxide ions than hydrogen ions. § This kind of solution is alkaline. § Acidity and alkalinity are measured with a logarithmic scale called p. H. 14 Dr Seemal Jelani 9/26/2020

p. H § p. H is the measure of acidity or alkalinity of a solution on a logarithmic scale on which 7 is neutral, lower values are more acid and higher values more alkaline. § The p. H is equal to −log 10 c, where c is the hydrogen ion concentration in moles per litre. § The p. H in the chemistry test used to test the acidity of substances stands for 'potential hydrogen, ' 15 Dr Seemal Jelani 9/26/2020

§ What are p. H levels? § why is it important? § The importance of p. H. The p. H level of water measures how acidic it is (p. H stands for potential hydrogen, referring to how much hydrogen is mixed with the water. ) 7 is a balanced p. H for water. Anything below 7 indicates the water is acidic, and if it's above 7 it is alkaline. 16 Dr Seemal Jelani 9/26/2020

What should your body p. H be? § Those levels vary throughout your body. § Your blood is slightly alkaline, with a p. H between 7. 35 and 7. 45. § Your stomach is very acidic, with a p. H of 3. 5 or below, so it can break down food. 17 Dr Seemal Jelani 9/26/2020

Why is p. H so important to life? § Acids and bases are important in living things because most enzymes can do their job only at a certain level of acidity. § Cells secrete acids and bases to maintain the proper p. H for enzymes to work. . § The acidic environment helps with the digestion of food 18 Dr Seemal Jelani 9/26/2020
What does a p. H of 5. 5 in urine mean? § A neutral p. H is 7. 0. The higher the number, the more basic (alkaline) it is. § The lower the number, the more acidic your urine is. § The average urine sample tests at about 6. 0. If your urine sample is lower, this could indicate an environment conducive to kidney stones. 19 Dr Seemal Jelani 9/26/2020
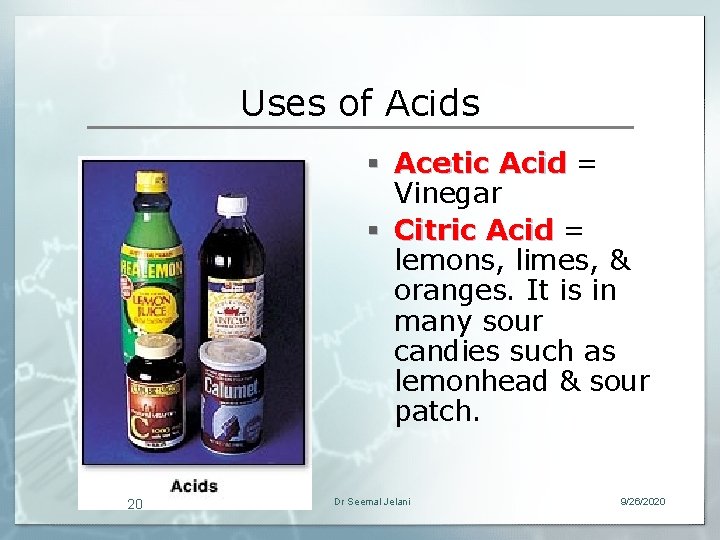
Uses of Acids § Acetic Acid = Vinegar § Citric Acid = lemons, limes, & oranges. It is in many sour candies such as lemonhead & sour patch. 20 Dr Seemal Jelani 9/26/2020

§ Ascorbic acid = Vitamin C which your body needs to function. § Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics. § Car batteries 21 Dr Seemal Jelani 9/26/2020

What is a base? base § A base is a solution that has an excess of OHions. § Another word for base is alkali. § Bases are Bases substances that can accept hydrogen ions 22 Dr Seemal Jelani 9/26/2020

§ Bases are bitter-tasting compounds with a slippery feel. § Most soaps and drainers and window cleaners are bases.

Properties of a Base Feel Slippery Taste Bitter Corrosive Can conduct electricity. (Think alkaline batteries. ) § Do not react with metals. § Turns red litmus paper blue. § § 24 Dr Seemal Jelani 9/26/2020

Uses of Bases § Bases give soaps, ammonia, and many other cleaning products some of their useful properties. 25 Dr Seemal Jelani 9/26/2020

§ The OH- ions interact strongly with certain substances, such as dirt and grease. § Chalk and oven cleaner are examples of familiar products that contain bases. § Your blood is a basic solution. 26 Dr Seemal Jelani 9/26/2020

Defining Indicators § Two of the most common indicators are phenolphthalein and litmus. § Litmus is a compound that is extracted from lichens, a plant-like member of the fungi kingdom § Litmus paper is made by dipping paper in litmus solution.
§ Chemical indicator, any substance that gives a visible sign, usually by a colour change. § What are indicators give examples? § Acid-Base Indicator Examples. § The best known p. H indicator is litmus. Thymol Blue, Phenol Red and Methyl Orange are all common acid-base indicators. § Red cabbage can also be used as an acidbase indicator 28

What are natural indicators give examples? § An acid (e. g. vinegar, lemon juice), purple in an alkali (e. g. bicarbonate of soda, bleach) and green in something that is neutral (e. g. water). § Many plants contain their own indicators – turmeric, red cabbage juice and beetroot juice are three good examples. Other examples are tea and red grape juice. 29 Dr Seemal Jelani 9/26/2020
How do indicators work in chemistry? § p. H indicators detect the presence of H+ and OH-. § They do this by reacting with H+ and OH-: they are themselves weak acids and bases. § If an indicator is a weak acid and is coloured and its conjugate base has a different colour, deprotonation causes a colour change. 30 Dr Seemal Jelani 9/26/2020

Some Common Indicators What are some examples of indicators? Name Acid Color p. H Range of Color Change Litmus Red 5. 0 - 8. 0 Bromothymol blue Yellow 6. 0 - 7. 6 Thymol blue Yellow 8. 0 - 9. 6 Phenolphthalein Colorless 8. 2 - 10. 0 31 Dr Seemal Jelani 9/26/2020

p. H Scale § p. H is a measure of how acidic or basic a solution is. • The p. H scale ranges from 0 to 14. § Acidic solutions have p. H values below 7 § A solution with a p. H of 0 is very acidic. § A solution with a p. H of 7 is neutral. • Pure water has a p. H of 7. 32 • Basic solutions have p. H values above 7. Dr Seemal Jelani 9/26/2020
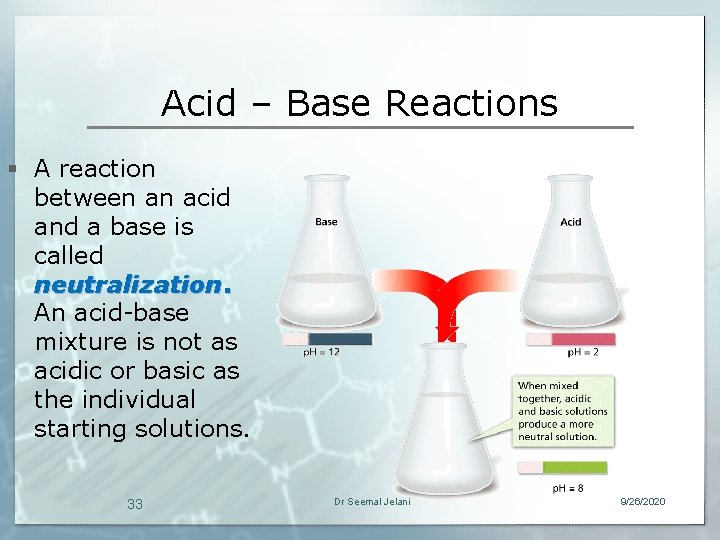
Acid – Base Reactions § A reaction between an acid and a base is called neutralization. An acid-base mixture is not as acidic or basic as the individual starting solutions. 33 Dr Seemal Jelani 9/26/2020
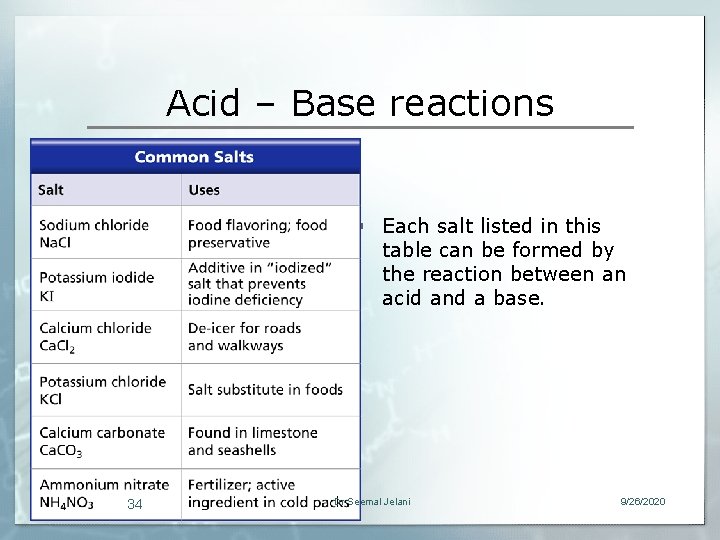
Acid – Base reactions § Each salt listed in this table can be formed by the reaction between an acid and a base. 34 Dr Seemal Jelani 9/26/2020
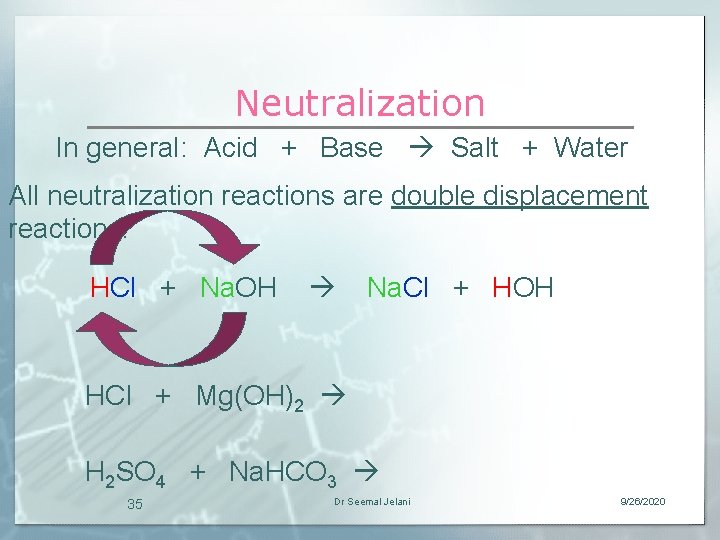
Neutralization In general: Acid + Base Salt + Water All neutralization reactions are double displacement reactions. HCl + Na. OH Na. Cl + HOH HCl + Mg(OH)2 H 2 SO 4 + Na. HCO 3 35 Dr Seemal Jelani 9/26/2020
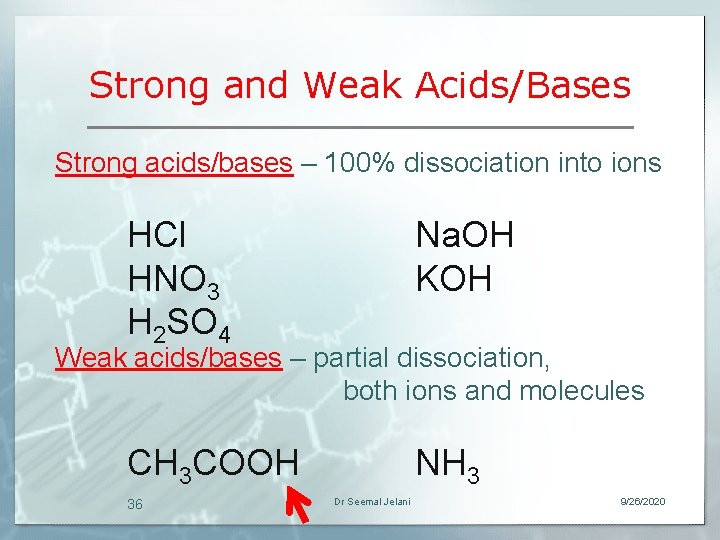
Strong and Weak Acids/Bases Strong acids/bases – 100% dissociation into ions HCl HNO 3 H 2 SO 4 Na. OH KOH CH 3 COOH NH 3 Weak acids/bases – partial dissociation, both ions and molecules 36 Dr Seemal Jelani 9/26/2020
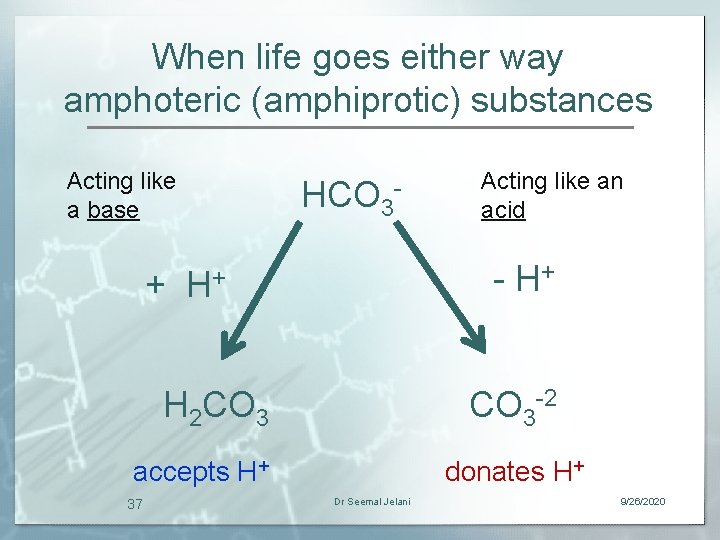
When life goes either way amphoteric (amphiprotic) substances Acting like a base HCO 3 - H+ H 2 CO 3 -2 + accepts H+ 37 Acting like an acid donates H+ Dr Seemal Jelani 9/26/2020
- Slides: 37