Technologies for cervical cancer detection diagnosis monitoring and























- Slides: 42

Technologies for cervical cancer detection, diagnosis, monitoring and treatment in low-resource settings 4 th WHO Global Forum on Medical Devices Visakhapatnam, Andhra Pradesh, India

Agenda § Background § Screen & Treat: technical guidance § Technical Guidance and Specifications – Diagnostics – Treatment § Next Steps 2|

Cervical Cancer - Disease Context § Primarily caused by persistent Human Papilloma Virus (HPV) infections. § HPV, a DNA virus, has over 100 documented genotypes – 40 of which are known to infect the anogenital tract. – Between 12 and 14 are “high risk” genotypes, those that can cause progression to cancer 1. § Cervical cancer is slow-growing. Its progression through precancerous changes provides opportunities for: 1. Prevention; 2. Early detection; and, 3. Treatment § Cervical cancer can be altogether eliminated. 3|

HPV and Cervical Cancer § Cervical cancer remains one of the gravest threats to women’s lives worldwide; § Cervical cancer is caused by high-risk types of HPV; – HPV 16 and 18: most common high-risk HPV types to cervical cancer – Responsible for approximately 70% of cervical cancer cases. § HPV is currently the most common sexually transmitted infection (STI) – 80% of women can be infected at some point in their lifetime; – Most of infection clear naturally 4|

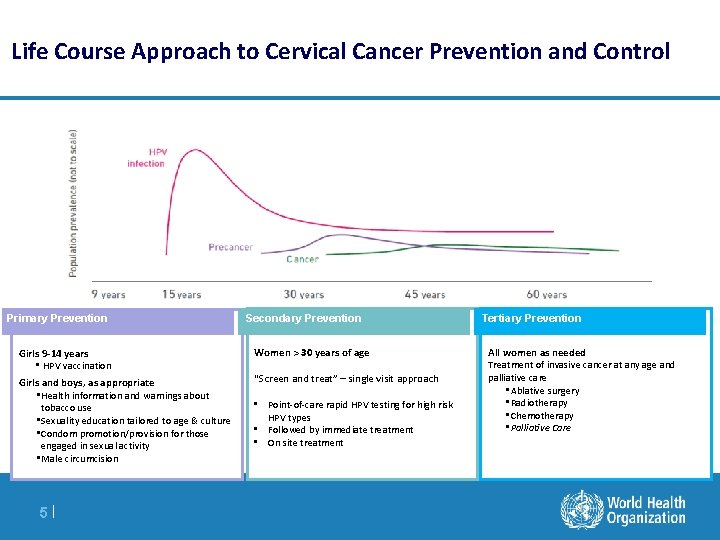
Life Course Approach to Cervical Cancer Prevention and Control Primary Prevention Girls 9 -14 years • HPV vaccination Girls and boys, as appropriate • Health information and warnings about tobacco use • Sexuality education tailored to age & culture • Condom promotion/provision for those engaged in sexual activity • Male circumcision 5| Secondary Prevention Women > 30 years of age “Screen and treat” – single visit approach • Point-of-care rapid HPV testing for high risk HPV types • Followed by immediate treatment • On site treatment Tertiary Prevention All women as needed Treatment of invasive cancer at any age and palliative care • Ablative surgery • Radiotherapy • Chemotherapy • Palliative Care

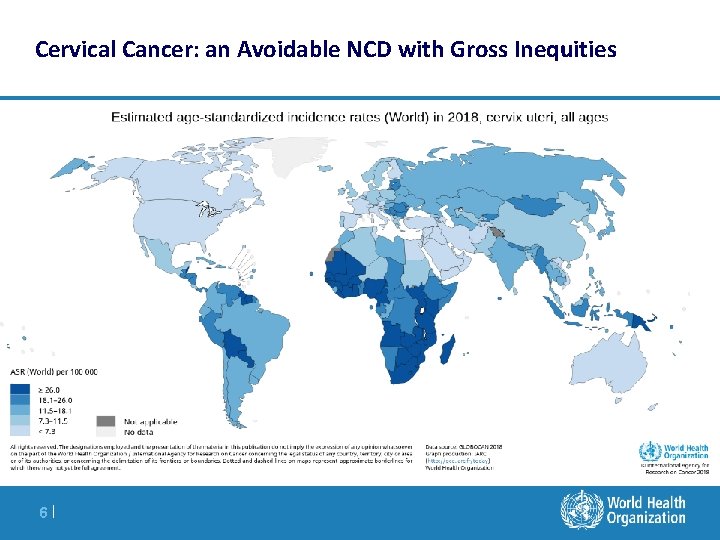
Cervical Cancer: an Avoidable NCD with Gross Inequities 6|

May 2018: WHO Director General’s Call to Action to Eliminate Cervical Cancer as a Public Health Problem 7|

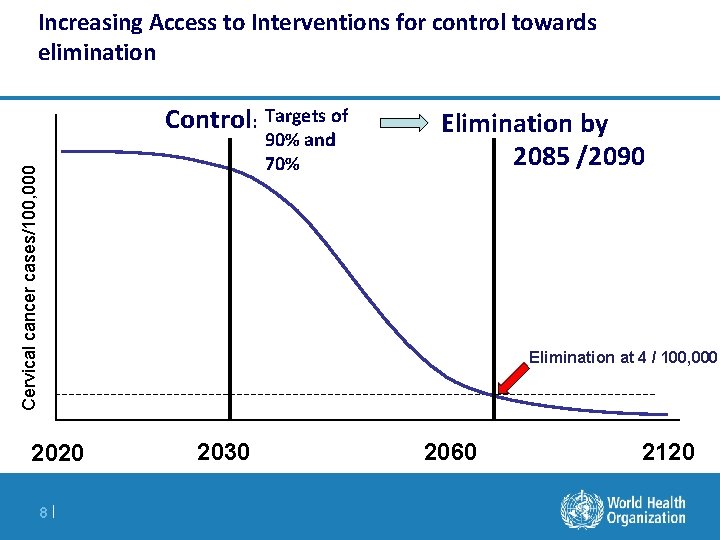
Increasing Access to Interventions for control towards elimination Control: Targets of Cervical cancer cases/100, 000 90% and 70% Elimination by 2085 /2090 Elimination at 4 / 100, 000 2020 8| 2030 2060 2120

2020 -2030 Acceleration plan towards elimination Vision: A world without cervical cancer 2030 TARGETS Goal: below 4 cases of cervical cancer per 100, 000 woman-years 90% 70% 30% of girls fully vaccinated with HPV vaccine by 15 years of age of women screened with an HPV test at 35 and 45 years of age and 90% managed appropriately reduction in mortality from cervical cancer The 2030 targets and elimination threshold are subject to revision depending on the outcomes of the modeling and the WHO approval process 9|

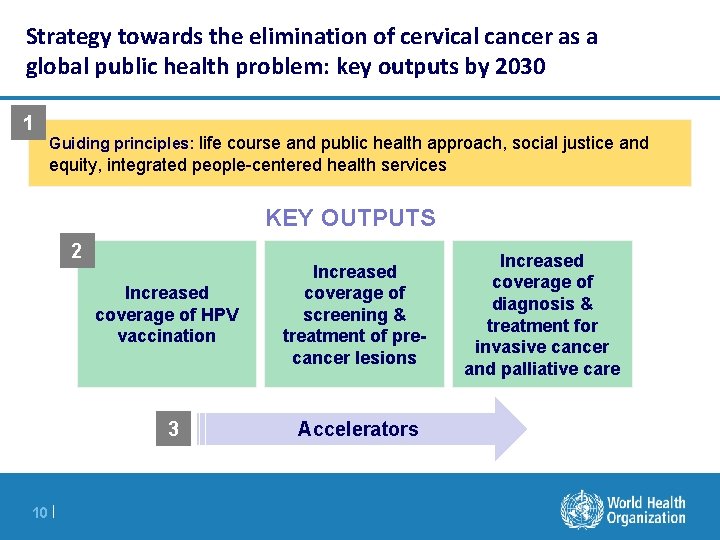
Strategy towards the elimination of cervical cancer as a global public health problem: key outputs by 2030 1 Guiding principles: life course and public health approach, social justice and equity, integrated people-centered health services KEY OUTPUTS 2 Increased coverage of HPV vaccination 3 10 | Increased coverage of screening & treatment of precancer lesions Accelerators Increased coverage of diagnosis & treatment for invasive cancer and palliative care

5. 1 Vaccinate: key component of the comprehensive prevention and control strategy § Two safe, effective vaccines that prevent infections from high-risk HPV types 16 and 18 are presently licensed in most countries. § One of the HPV vaccines (quadri- and nono-valent), also prevents infections from HPV types 6 and 11, which cause 90% of anogenital warts Target: 90% of girls fully vaccinated w HPV vaccine by 15 years of age – Introduced in 85 countries by October 2018 – Coverage varies between less than 10% and more than 90% § Vaccines do not cover all cancer-causing HPV types and do not treat preexisting infections; thus, ongoing screening is mandatory. § Accelerated focus needed in Africa and Southern Asia *2030 targets & elimination threshold are subject to revision depending on outcomes of the modeling & the WHO approval process. 11 |

5. 2 Screen & Treat: reduces loss to follow-up, and can reduce the time lag for women to receive treatment § Early detection by screening all women in the target age-group, followed by treatment of detected precancerous lesions, can prevent the majority of cervical cancers. § Cervical cancer screening should be performed at least once for every woman in the target age group where most benefit can be achieved. Target: 70% of women screened with an HPV test at 35 and 45 years of age & 90% of the one screened positive managed appropriately § Devices that can detect HPV, cytology and VIA play an important role in cervical cancer prevention programs. § Accordingly, effective and appropriate technologies for the treatment of precancerous lesions be used: LEEP, cryotherapy, or thermal ablation. *2030 targets & elimination threshold are subject to revision depending on outcomes of the modeling & the WHO approval process. 12 |

5. 3 Invasive Cancer Management § Women diagnosed with early invasive cervical cancer can usually be cured with effective treatment. § It is important for health-care providers at all levels to be able to recognize and promptly manage common symptoms and signs of cervical cancer. § The definitive diagnosis of invasive cervical cancer is made by histopathological examination of a biopsy. Target: 30% reduction of mortality from cervical cancer § Treatment options include surgery, radiotherapy and chemotherapy; these may be used in combination. If left untreated, invasive cervical cancer is almost always fatal. 13 |

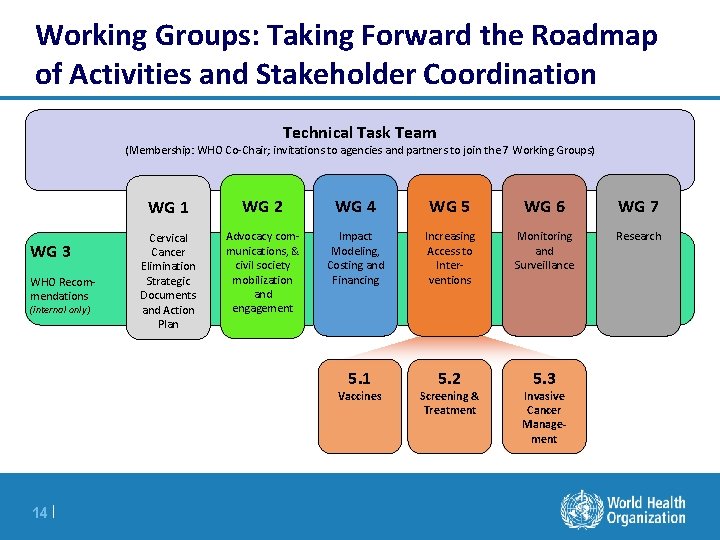
Working Groups: Taking Forward the Roadmap of Activities and Stakeholder Coordination Technical Task Team (Membership: WHO Co-Chair; invitations to agencies and partners to join the 7 Working Groups) WG 3 WHO Recommendations (internal only) WG 1 WG 2 WG 4 WG 5 WG 6 WG 7 Cervical Cancer Elimination Strategic Documents and Action Plan Advocacy communications, & civil society mobilization and engagement Impact Modeling, Costing and Financing Increasing Access to Interventions Monitoring and Surveillance Research 5. 1 5. 2 5. 3 Vaccines 14 | Screening & Treatment Invasive Cancer Management

Agenda § Background § Screen & Treat: technical guidance § Technical Guidance and Specifications Chapters – Diagnostics – Treatment § Next Steps 15 |

Screen & Treat Technical guidance and specifications - why? § A key gap identified was the lack of technical guidance and specifications for the majority of cervical cancer related tests and treatment technologies. § Technical specifications and guidance documents are critical in facilitating the procurement, supply, or use of quality, appropriate technologies. § A strong focus of these documents will be the application in resourcelimited settings where existing guidance (if at all) is limited or contextually unsuitable. 16 |

Screen & Treat Technical guidance and specifications – what? § Current efforts will cover technical guidance and specifications for the diagnostics and treatment of precancerous lesions for the prevention of cervical cancer in terms of: – Quality – Performance – Operational characteristics 17 |

Screen & Treat Technical guidance and specifications – how? § These will be useful for Policymakers, Managers, Procurement officers, Professional health workers, and even manufacturers for: – Procuring; – Supplying; or, – Using devices for treating cervical precancer. § These documents will fit into the WHO repository of resources, to be used alongside: 18 |

Agenda § Background § Screen & Treat: technical guidance § Technical Guidance and Specifications Chapters – Diagnostics – Treatment § Next Steps 19 |

Outline for the forthcoming Technical Guidance and specification document – Thermal Ablation – Cryotherapy – LEEP § Section 3: Additional information Medical devices for the diagnosis and treatment of pre-cancerous lesions for the prevention of cervical cancer FT § Section 2: Treatment WHO technical guidance & specifications RA – HPV NAT IVD – VIA – Colposcope D § Section 1: Screening and Diagnosis – WHO Infection Prevention and Control – Procurement guidance – Technical knowledge gaps or areas identified for further research 20 |

HPV NAT IVD (1 of 4): Background § Because HPV is the causative agent of cervical cancer, detection of HPV has the potential to improve cervical cancer prevention programs § HPV is a relatively small double stranded DNA virus that is present and accessible in infected exfoliated cell specimens, allowing detection by molecular nucleic acid tests (NAT) that utilize primers and probes to amplify DNA or RNA targets § HPV NATs for qualitative detection of high risk genotypes cover a range of specifications: – DNA and RNA-based technologies • rt. PCR, probe hybridization, invader signal, microarray PCR, TMA, NASBA – Reporting out pooled and/or individual genotypes – Internal controls for sample adequacy and to rule out inhibitory substances – Manual, high throughput automated and point-of-care methodology 21 |

HPV NATs (2 of 4): Considerations When Implementing § Specimen acquisition: – Health-care provider collected • Need for exam table/light, specula, gloves, sterilization equipment – Self-collected • In clinic or home-based § Performance of NATs: – Laboratory or point-of-care – Infrastructure and equipment considerations • Are electricity, running water, refrigeration/freezing required? • Possible need for specimen racks, pipettes/tips, centrifuge, heating blocks, disinfectants – Workflow: batch or single samples, throughput requirements § Interpretation of Results – Health-care provider training in interpretation and next steps essential 22 |

HPV NATs (3 of 4): Strengths and Limitations § HPV NATs have the potential to improve cervical cancer prevention programs by improving sensitivity of precancer detection § Provide objective screening results § Allow for self-collected sampling § Workflow flexibility: – Laboratory- vs clinic-based – Manual, automated or point-of-care platforms § Infrastructure requirements and equipment may not be accessible in LRS § Need for trained health-care providers and laboratory personnel 23 |

HPV NAT IVD (4 of 4): Technical Specification Guidance § Clinical Performance: – At least 90% of sensitivity of the designated comparator for detection of CIN 2 or greater – Not less than 98% of specificity of the designated comparator – lower bound of agreement not less than 87% for inter- and intra-laboratory reproducibility § Analytic performance: – Sensitivity: (Limit of Detection) dependent on genotype detected, characteristics of particular test – Specificity: cross-reactivity with a panel of organisms (to include those common to female urogenital tract) and low risk HPV genotypes; assessment of effect of endogenous and exogenous interfering substances in cervical specimens § Operational requirements – Infrastructure, equipment, specimen throughput, reagent storage, waste disposal: key considerations that are dependent on particular test and need to be addressed 24 |

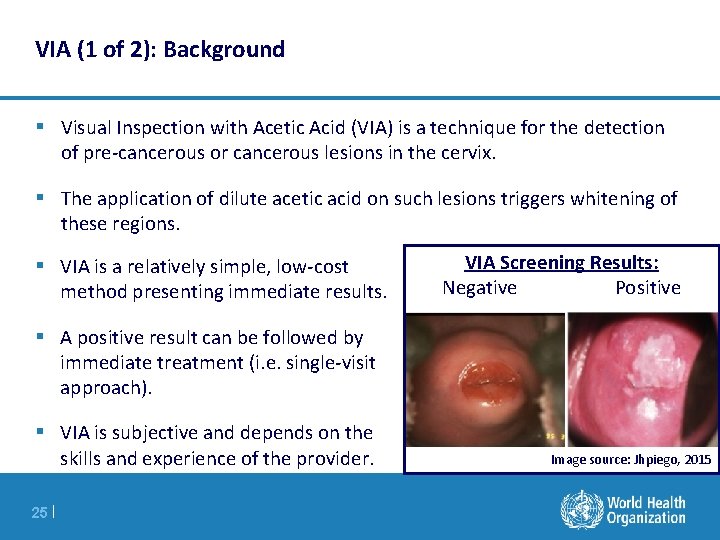
VIA (1 of 2): Background § Visual Inspection with Acetic Acid (VIA) is a technique for the detection of pre-cancerous or cancerous lesions in the cervix. § The application of dilute acetic acid on such lesions triggers whitening of these regions. § VIA is a relatively simple, low-cost method presenting immediate results. VIA Screening Results: Negative Positive § A positive result can be followed by immediate treatment (i. e. single-visit approach). § VIA is subjective and depends on the skills and experience of the provider. 25 | Image source: Jhpiego, 2015

VIA (2 of 2): Technical Specification Guidance § 26 |

Colposcope (1 of 3): Background § A colposcope is an instrument that provides strong light and magnifies a field, allowing specific patterns in the epithelial (surface) layer and surrounding blood vessels to be examined. § Colposcopes are used on patients with positive screening results, to: – verify the presence, extent, and type of pre-cancer or cancer; – to guide biopsies of any areas that appear abnormal; and, – to help determine more appropriate treatment (cryotherapy, LEEP, or TA). § Colposcopy requires highly trained providers and is not an appropriate screening tool, nor is colposcopy a required step between screening and treatment. § More recently, colposcopes are being designed as handheld, specialized video or digital camera tools. 27 |

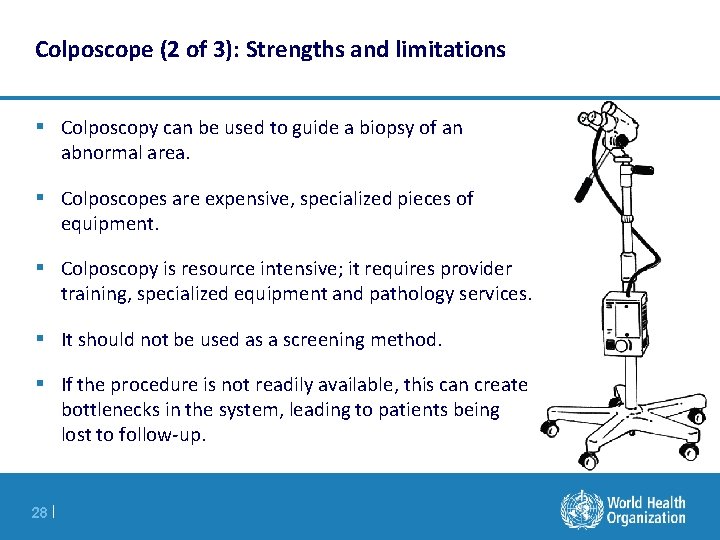
Colposcope (2 of 3): Strengths and limitations § Colposcopy can be used to guide a biopsy of an abnormal area. § Colposcopes are expensive, specialized pieces of equipment. § Colposcopy is resource intensive; it requires provider training, specialized equipment and pathology services. § It should not be used as a screening method. § If the procedure is not readily available, this can create bottlenecks in the system, leading to patients being lost to follow-up. 28 |

Colposcope (3 of 3): Technical Specification Guidance § Optical magnification: 2 x-15 x, continuous or discrete. § Working distance: 300 mm. § Manual or autofocus. § Colposcope head including eyepiece distance should be high and maneuverable. § Must be attached to a stand or easily fixed onto a stand for hands-free visualization during treatment. § Light source: halogen or LED, with green light filter. 29 |

Thermal Ablation (1 of 3): Background § Thermal ablation has been used effectively in some settings for many years, especially for the treatment of endometriosis. § Thermal ablation uses low heat to destroy lesions. § It has limited side-effects, is inexpensive compared with other treatment options, it is not dependent on a continuous supply chain, and is technically simple to implement. § Only recently have handheld devices come on the market, making the method more suitable for LRS (benchtop models are cumbersome and reliant on mains, though highly effective). § Thermal ablation can be performed immediately after screening, by a range of trained healthcare providers across all levels of health systems. 30 |

Thermal Ablation (2 of 3): Strengths and limitations § The equipment is simple & relatively inexpensive. § External gas is not required. § Electricity is not necessarily required; can function off of portable batteries/power-supply. § In the context of a screen & treat approach, a screen-positive result can be followed by an offer of treatment at the same visit, maximizing treatment coverage and reducing loss to follow-up. § This treatment method does not produce a specimen for pathological examination. 31 |

Thermal Ablation (3 of 3): Technical Specification Guidance § Handheld (rechargeable) or benchtop (plug-in) § Compatible for use with at least two probes with different-sized tips – Standard tip diameters: 16 mm and 19 mm – A flat tip and a tip with a nipple for placement into the cervical canal § Heat to 100 -120°C and has a depth penetration of 4 -7 mm § Typical duty cycle: – 8 seconds of heat up; – 20 -45 seconds of treatment; and – 10 seconds to cool down. § Tips should be easily decontaminated, cleaned, and sterilized or disinfected between patients. 32 |

Cryotherapy (1 of 3): Background § Cryotherapy eliminates precancerous areas on the cervix by applying a highly cooled metal disc (cryoprobe) to the cervix and freezing the abnormal areas (along with normal areas) covered by it. § Supercooling of the cryoprobe is accomplished using a coolant gas (either compressed carbon dioxide, CO 2, or nitrous oxide, N 2 O), thus relies on a complex supply chain. § Cryotherapy can be performed immediately after screening, usually by a wide range of trained health care providers across all levels of the health system, and has long been the standard in LRS. § It takes ~15 minutes and is generally well tolerated, associated with only mild discomfort, and thus does not require anaesthesia. The treated area takes about a month to regenerate. 33 |

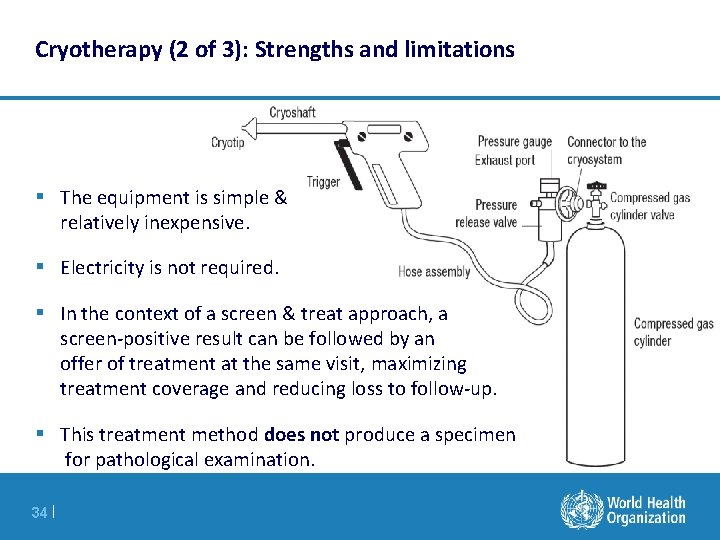
Cryotherapy (2 of 3): Strengths and limitations § The equipment is simple & relatively inexpensive. § Electricity is not required. § In the context of a screen & treat approach, a screen-positive result can be followed by an offer of treatment at the same visit, maximizing treatment coverage and reducing loss to follow-up. § This treatment method does not produce a specimen for pathological examination. 34 |

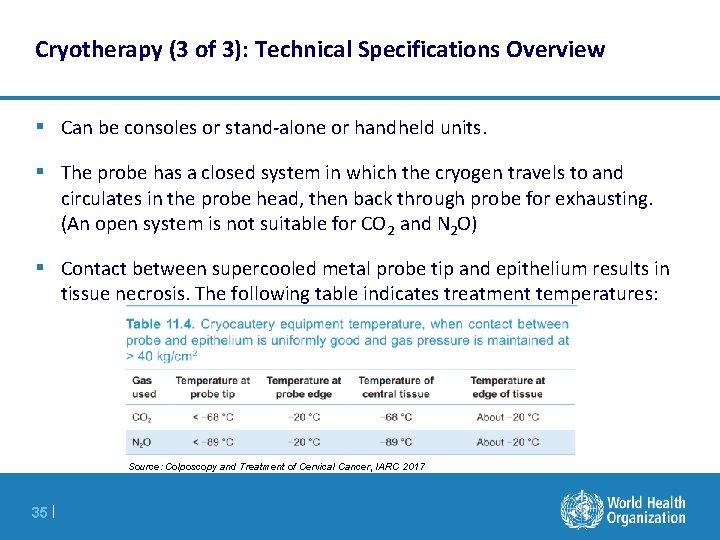
Cryotherapy (3 of 3): Technical Specifications Overview § Can be consoles or stand-alone or handheld units. § The probe has a closed system in which the cryogen travels to and circulates in the probe head, then back through probe for exhausting. (An open system is not suitable for CO 2 and N 2 O) § Contact between supercooled metal probe tip and epithelium results in tissue necrosis. The following table indicates treatment temperatures: Source: Colposcopy and Treatment of Cervical Cancer, IARC 2017 35 |

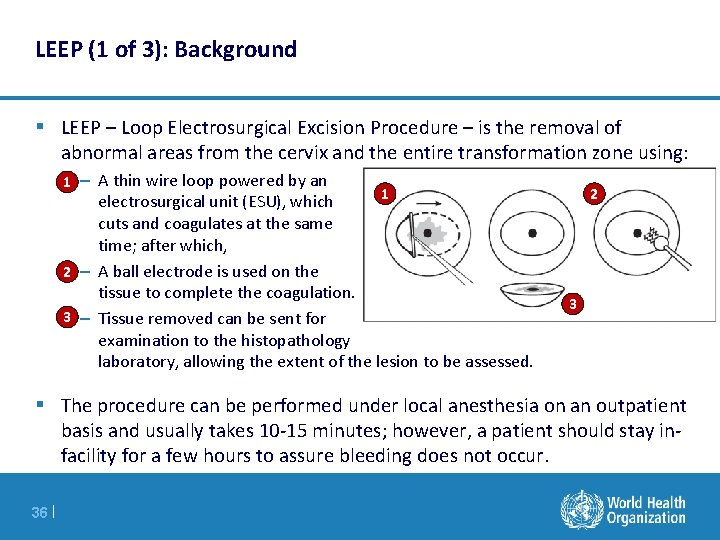
LEEP (1 of 3): Background § LEEP – Loop Electrosurgical Excision Procedure – is the removal of abnormal areas from the cervix and the entire transformation zone using: 1 – A thin wire loop powered by an 1 electrosurgical unit (ESU), which cuts and coagulates at the same time; after which, 2 – A ball electrode is used on the tissue to complete the coagulation. 3 – Tissue removed can be sent for examination to the histopathology laboratory, allowing the extent of the lesion to be assessed. 2 3 § The procedure can be performed under local anesthesia on an outpatient basis and usually takes 10 -15 minutes; however, a patient should stay infacility for a few hours to assure bleeding does not occur. 36 |

LEEP (2 of 3): Strengths and limitations § LEEP is a relatively simple surgical procedure, but it should only be performed by an intensively trained health-care provider with demonstrated competence in the procedure § Recognizing and managing intraoperative and postoperative complications, such as bleeding or infection is crucial; LEEP is best carried out in at least secondary-level facilities where backup care is available. § The histology specimen can have charred borders, making lesion margins difficult to interpret. § LEEP: – Relies on dependable, quality power supply – Uses sophisticated equipment that requires maintenance. 37 |

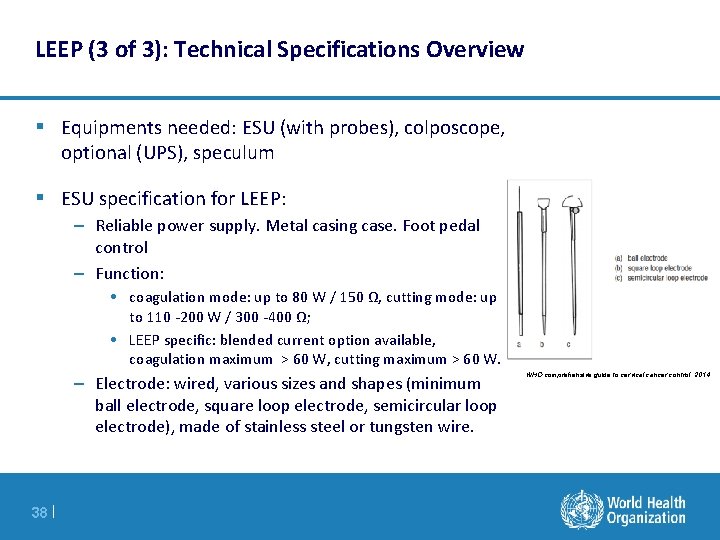
LEEP (3 of 3): Technical Specifications Overview § Equipments needed: ESU (with probes), colposcope, optional (UPS), speculum § ESU specification for LEEP: – Reliable power supply. Metal casing case. Foot pedal control – Function: • coagulation mode: up to 80 W / 150 Ω, cutting mode: up to 110 -200 W / 300 -400 Ω; • LEEP specific: blended current option available, coagulation maximum > 60 W, cutting maximum > 60 W. – Electrode: wired, various sizes and shapes (minimum ball electrode, square loop electrode, semicircular loop electrode), made of stainless steel or tungsten wire. 38 | WHO comprehensive guide to cervical cancer control, 2014

Agenda § Background § Screen & Treat: technical guidance § Technical Guidance and Specifications Chapters – Diagnostics – Treatment § Next Steps 39 |

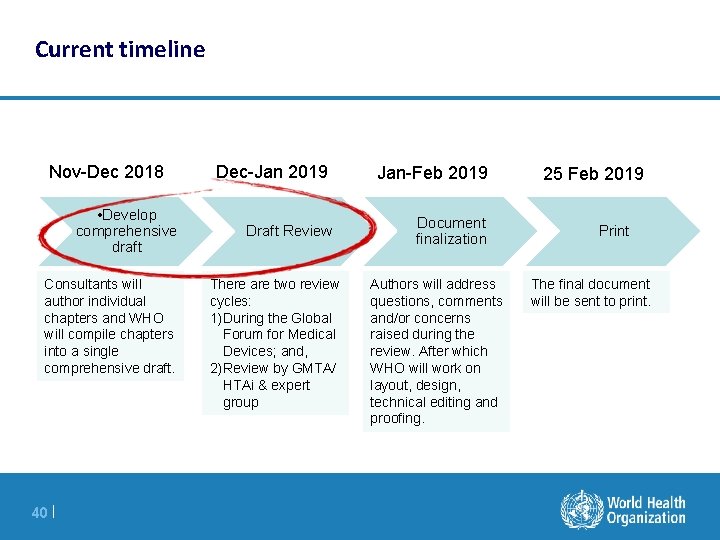
Current timeline Nov-Dec 2018 • Develop comprehensive draft Consultants will author individual chapters and WHO will compile chapters into a single comprehensive draft. 40 | Dec-Jan 2019 Jan-Feb 2019 Draft Review Document finalization There are two review cycles: 1)During the Global Forum for Medical Devices; and, 2)Review by GMTA/ HTAi & expert group Authors will address questions, comments and/or concerns raised during the review. After which WHO will work on layout, design, technical editing and proofing. 25 Feb 2019 Print The final document will be sent to print.

Output: A new technical guidance and specification document! 41 |

Next steps § To follow will be technical guidance and specifications for diagnostics and technologies for invasive cervical cancer treatment: – Surgical procedures; – Radiotherapy; and, – Chemotherapy. 42 |
 National breast and cervical cancer early detection program
National breast and cervical cancer early detection program National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Kolposkopi px
Kolposkopi px Stage 4 cervical cancer
Stage 4 cervical cancer Hpv cervical cancer
Hpv cervical cancer Hpv cervical cancer
Hpv cervical cancer Cervical cancer hcp
Cervical cancer hcp Cervical myelopathy wayne
Cervical myelopathy wayne What is nursing process
What is nursing process Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis three parts
Nursing diagnosis three parts Objectives of nursing process
Objectives of nursing process Mylosuppresion
Mylosuppresion Smart goals for ineffective airway clearance
Smart goals for ineffective airway clearance Frozen section
Frozen section Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Babinski sign
Babinski sign L4 nerve path
L4 nerve path Spinal cord and spinal nerves exercise 15
Spinal cord and spinal nerves exercise 15 Difference between cervical thoracic and lumbar vertebrae
Difference between cervical thoracic and lumbar vertebrae Fspos
Fspos Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok yrkesförare
Tidbok yrkesförare Sura för anatom
Sura för anatom Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Hur skriver man en tes
Hur skriver man en tes Delegerande ledarskap
Delegerande ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Arkimedes princip formel
Arkimedes princip formel Offentlig förvaltning
Offentlig förvaltning Urban torhamn
Urban torhamn Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Plats för toran ark
Plats för toran ark Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter