Structure Prediction Tertiary protein structure protein folding Three
![Tertiary protein structure: protein folding Three main approaches: [1] experimental determination (X-ray crystallography, NMR) Tertiary protein structure: protein folding Three main approaches: [1] experimental determination (X-ray crystallography, NMR)](https://slidetodoc.com/presentation_image_h2/10d73df5d396baf0835dbb0c46dfa7c6/image-2.jpg)
![Experimental approaches to protein structure [1] X-ray crystallography -- Used to determine 80% of Experimental approaches to protein structure [1] X-ray crystallography -- Used to determine 80% of](https://slidetodoc.com/presentation_image_h2/10d73df5d396baf0835dbb0c46dfa7c6/image-3.jpg)



- Slides: 29

Structure Prediction
![Tertiary protein structure protein folding Three main approaches 1 experimental determination Xray crystallography NMR Tertiary protein structure: protein folding Three main approaches: [1] experimental determination (X-ray crystallography, NMR)](https://slidetodoc.com/presentation_image_h2/10d73df5d396baf0835dbb0c46dfa7c6/image-2.jpg)
Tertiary protein structure: protein folding Three main approaches: [1] experimental determination (X-ray crystallography, NMR) [2] Comparative modeling (based on homology) [3] Ab initio (de novo) prediction (Dr. Ingo Ruczinski at JHSPH)
![Experimental approaches to protein structure 1 Xray crystallography Used to determine 80 of Experimental approaches to protein structure [1] X-ray crystallography -- Used to determine 80% of](https://slidetodoc.com/presentation_image_h2/10d73df5d396baf0835dbb0c46dfa7c6/image-3.jpg)
Experimental approaches to protein structure [1] X-ray crystallography -- Used to determine 80% of structures -- Requires high protein concentration -- Requires crystals -- Able to trace amino acid side chains -- Earliest structure solved was myoglobin [2] NMR -- Magnetic field applied to proteins in solution -- Largest structures: 350 amino acids (40 k. D) -- Does not require crystallization

Steps in obtaining a protein structure Target selection Obtain, characterize protein Determine, refine, model the structure Deposit in database

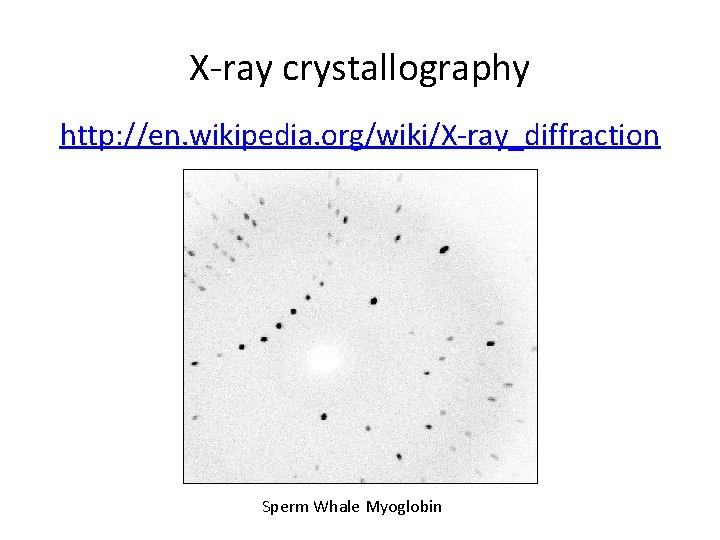
X-ray crystallography http: //en. wikipedia. org/wiki/X-ray_diffraction Sperm Whale Myoglobin



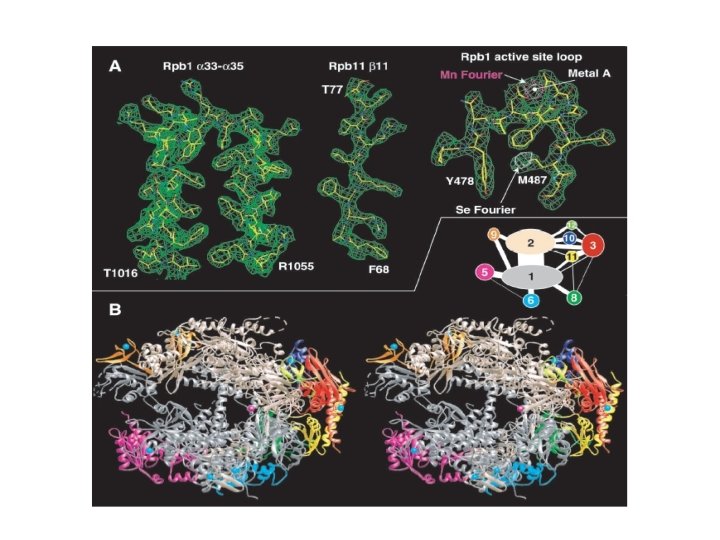
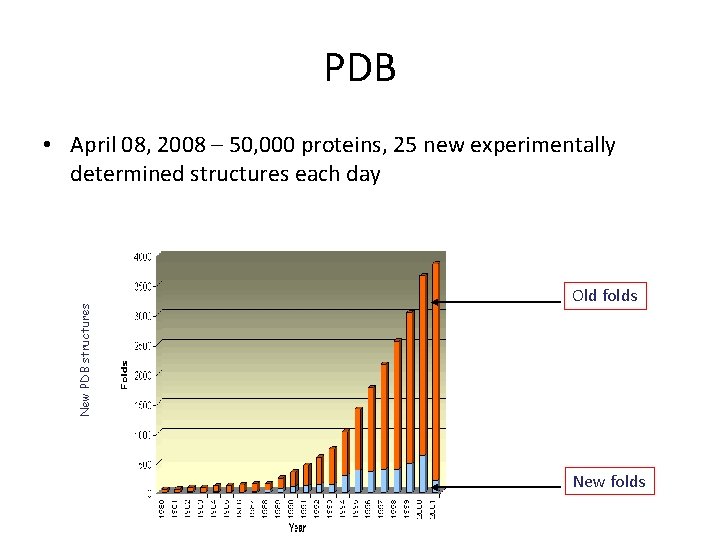
PDB New PDB structures • April 08, 2008 – 50, 000 proteins, 25 new experimentally determined structures each day Old folds New folds

Example 1 wey

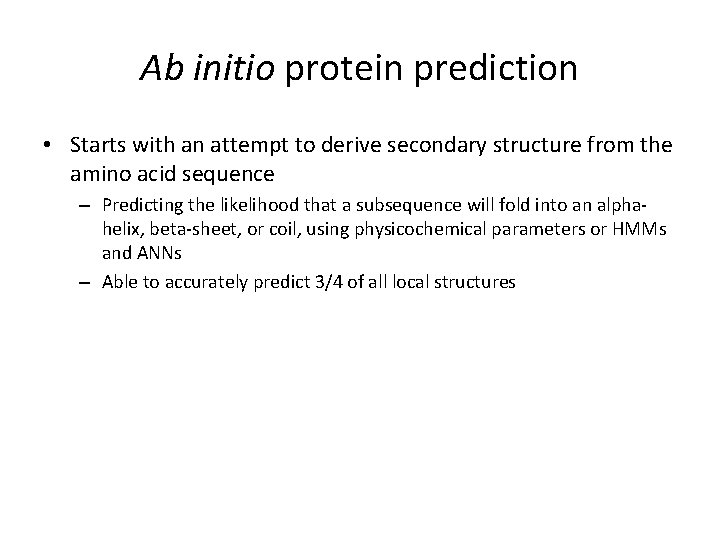
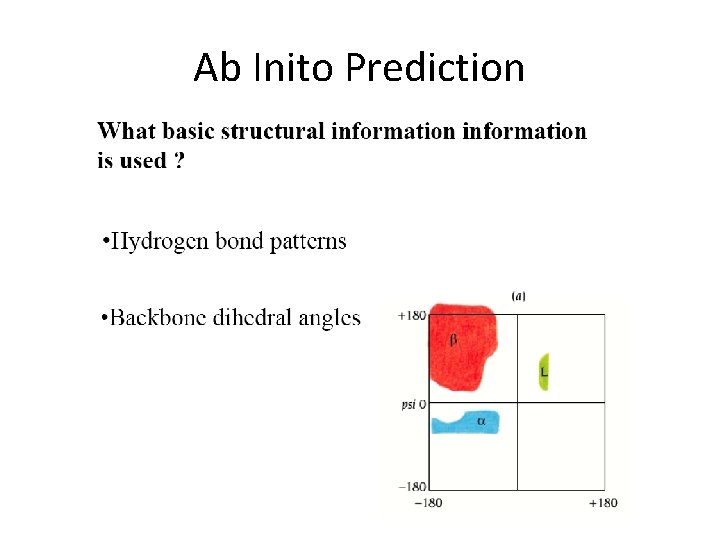
Ab initio protein prediction • Starts with an attempt to derive secondary structure from the amino acid sequence – Predicting the likelihood that a subsequence will fold into an alphahelix, beta-sheet, or coil, using physicochemical parameters or HMMs and ANNs – Able to accurately predict 3/4 of all local structures

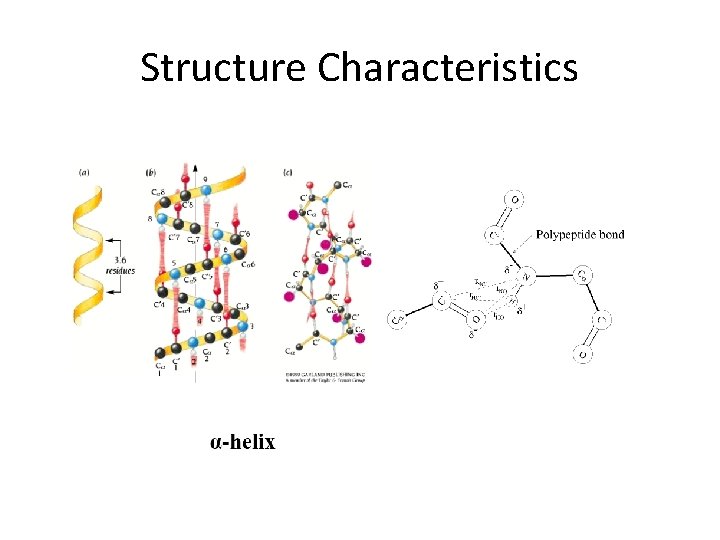
Structure Characteristics

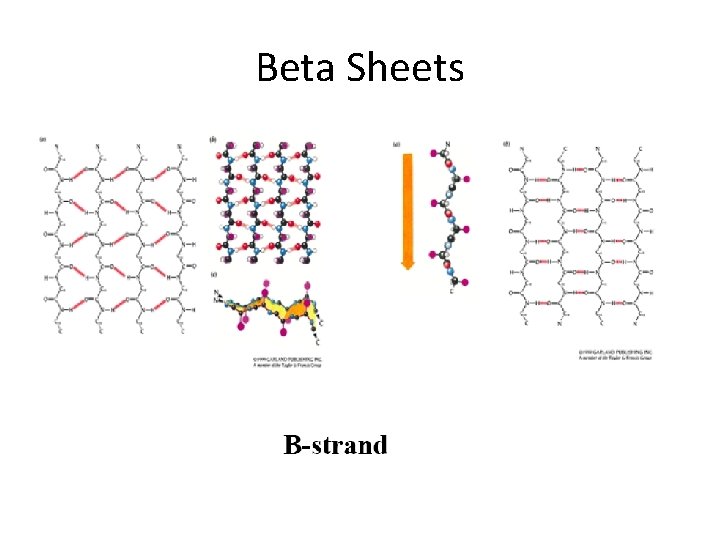
Beta Sheets

Ab Inito Prediction

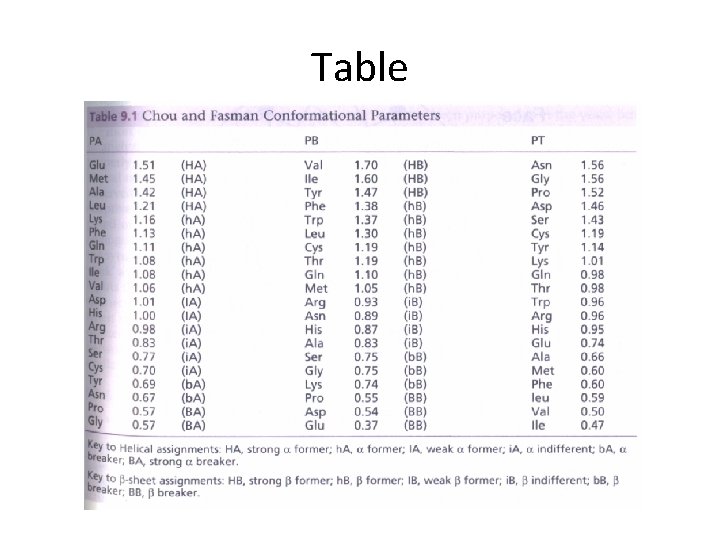
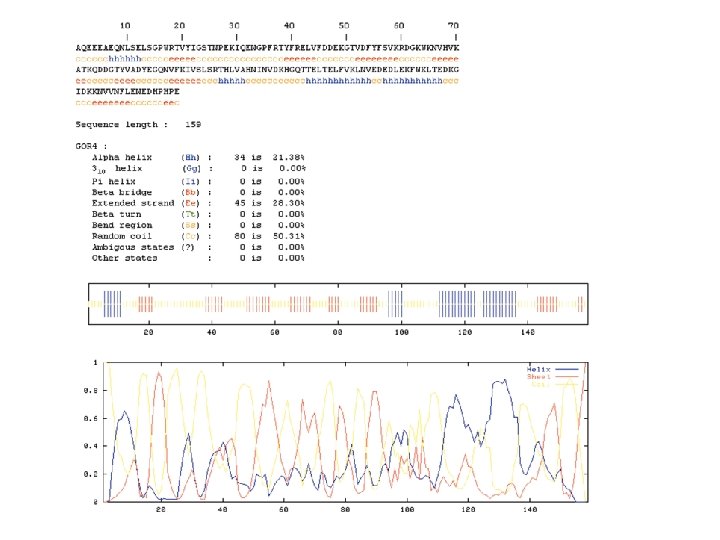
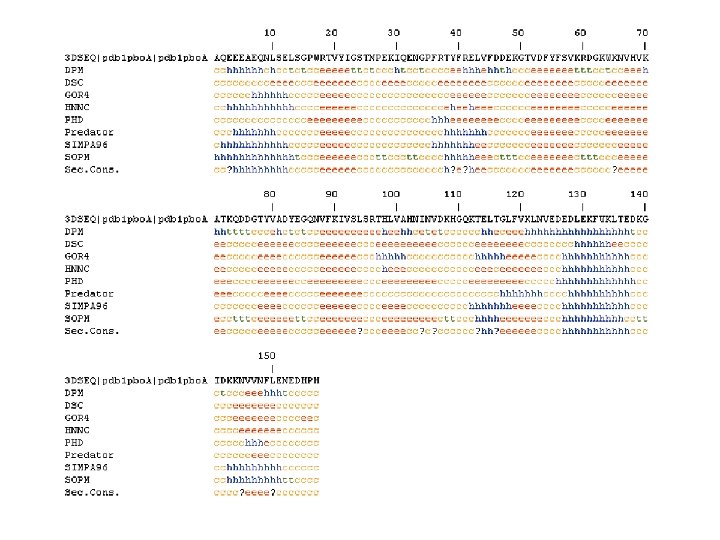
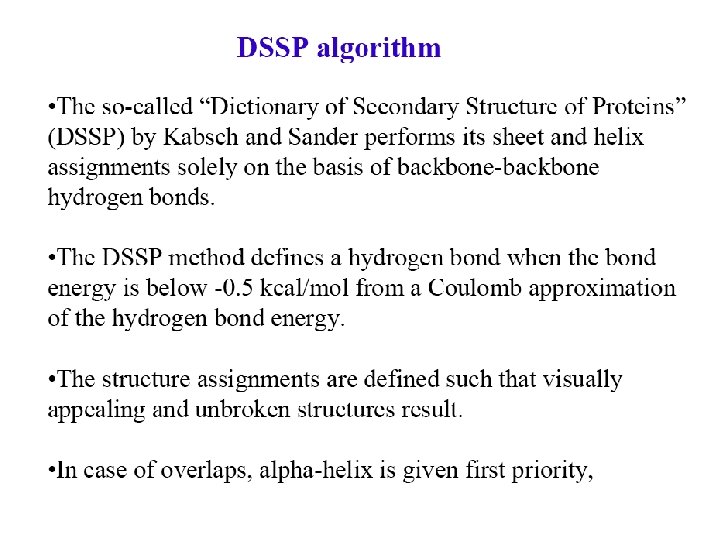
Secondary structure prediction Chou and Fasman (1974) developed an algorithm based on the frequencies of amino acids found in a helices, b-sheets, and turns. Proline: occurs at turns, but not in a helices. GOR (Garnier, Osguthorpe, Robson): related algorithm Modern algorithms: use multiple sequence alignments and achieve higher success rate (about 70 -75%) Page 279 -280

Table




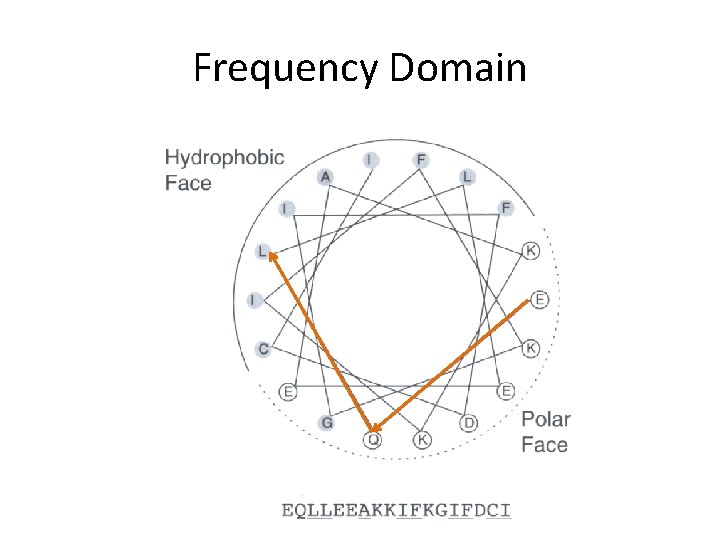
Frequency Domain

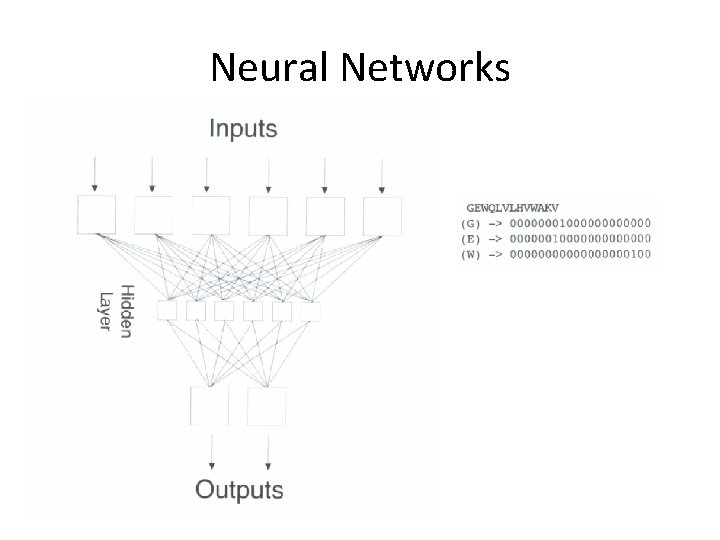
Neural Networks

Training the Network • Use PDB entries with validated secondary structures • Measures of accuracy – Q 3 Score percentage of protein correctly predicted (trains to predicting the most abundant structure) – You get 50% if you just predict everything to be a coil – Most methods get around 60% with this metric

Correlation Coeficient • How correlated are the predictions for coils, helix and Beta-sheets to the real structures • This ignores what we really want to get to – If the real structure has 3 coils, do we predict 3 coils? • Segment overlap score (Sov) gives credit to how protein like the structure is, but it is correlated with Q 3


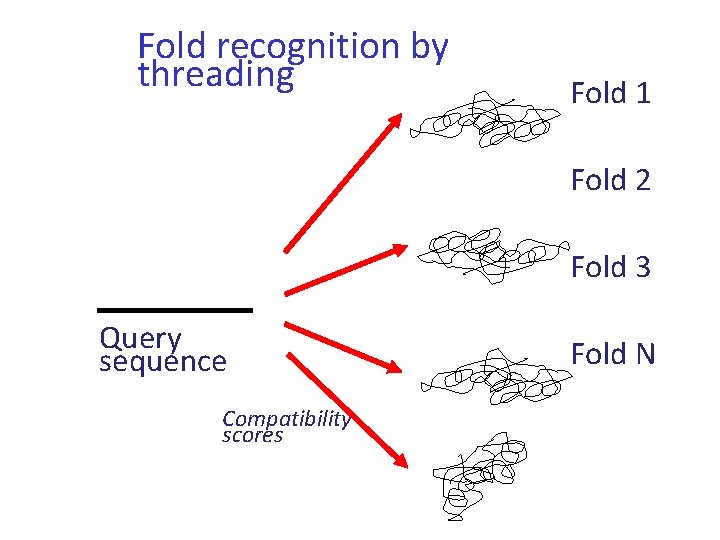
Fold recognition (structural profiles) • Attempts to find the best fit of a raw polypeptide sequence onto a library of known protein folds • A prediction of the secondary structure of the unknown is made and compared with the secondary structure of each member of the library of folds

Threading • Takes the fold recognition process a step further: – Empirical-energy functions for residue pair interactions are used to mount the unknown onto the putative backbone in the best possible manner

Fold recognition by threading Fold 1 Fold 2 Fold 3 Query sequence Compatibility scores Fold N

CASP • http: //www. predictioncenter. org/casp 8/index. cgi

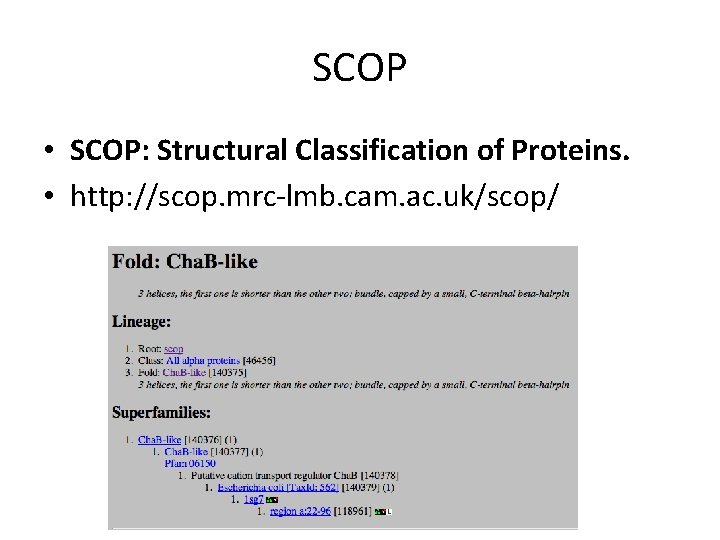
SCOP • SCOP: Structural Classification of Proteins. • http: //scop. mrc-lmb. cam. ac. uk/scop/

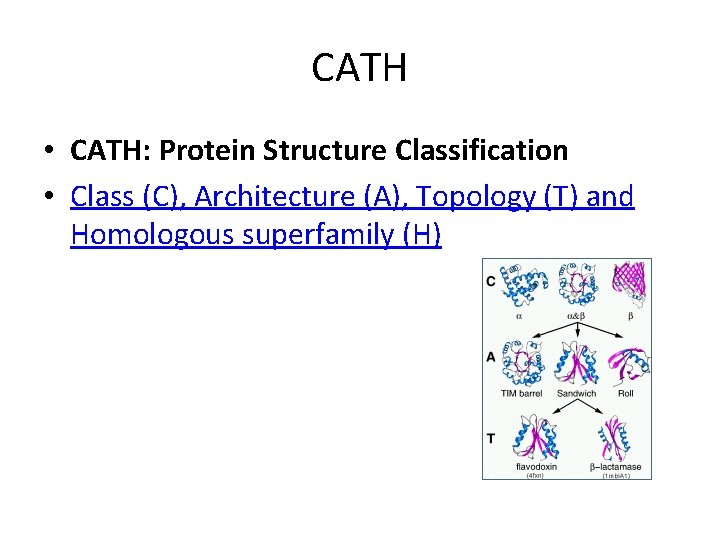
CATH • CATH: Protein Structure Classification • Class (C), Architecture (A), Topology (T) and Homologous superfamily (H)