PROTEINS FOLDED POLYPEPTIDES 2007 Paul Billiet ODWS WarmUp
PROTEINS FOLDED POLYPEPTIDES © 2007 Paul Billiet ODWS

Warm-Up n Draw the structure of glycerol. n How many fatty acids will combine with glycerol to make a lipid? n Is lactose a monosaccharide, disaccharide or polysaccaride?

Day 4 - Amino Acids and Proteins n GOAL: SWABAT identify amino acids and explain their properties. n Homework: Flashcards 24 -25, Packet pgs. 11 -14. n Mid-Term Exam: Tuesday, July 23 rd!


AMINO ACIDS & PROTEINS: C, H, O, N, S cysteine arginine methionine aspartic acid © 2007 Paul Billiet ODWS phenylalaline

Amino acids n amino group, carboxyl group, hydrogen and a variable side group (residue) each joined to a central carbon atom R H 2 N-C-COOH H © 2007 Paul Billiet ODWS

Types of amino acids Amino end and carboxyl end can be ionized to –NH 3+ and -COO- to give acidic and basic characteristics n At p. H 7 both groups are ionized. n The residues are side chains which give the individual properties to the amino acid (acidic, basic, neutral and nonpolar) n © 2007 Paul Billiet ODWS

Functions of amino acids Protein synthesis, energy reserve, hormones (thyroxin) n 20 different amino acids used in protein synthesis though others do occur in nature. n Essential amino acids cannot be synthesised by the organism and must form part of their diet n © 2007 Paul Billiet ODWS

The peptide bond Carboxyl group + amino group form a strong covalent bond releasing water in to process water = a condensation reaction (the reverse is hydrolysis) n Amino acids join together in a long chain: N terminal end to C terminal end = a polypeptide n © 2007 Paul Billiet ODWS

H H N R O C C-OH H H N H R O C C-OH H Condensation reaction A dipeptide is formed H H N R O H R O C C N C C-OH H H The peptide bond © 2007 Paul Billiet ODWS + H 2 O


Introduction n Proteins are instrumental in about everything that an organism does. ¨ These functions include structural support, storage, transport of other substances, intercellular signaling, movement, and defense against foreign substances. ¨ Proteins are the overwhelming enzymes in a cell and regulate metabolism by selectively accelerating chemical reactions. n Humans have tens of thousands of different proteins, each with their own structure and function. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n Proteins are the most structurally complex molecules known. ¨ Each type of protein has a complex threedimensional shape or conformation. All protein polymers are constructed from the same set of 20 monomers, called amino acids. n Polymers of proteins are called polypeptides. n A protein consists of one or more polypeptides folded and coiled into a specific conformation. n Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

1. A polypeptide is a polymer of amino acids connected in a specific sequence Amino acids consist of four components attached to a central carbon, the alpha carbon. n These components include a hydrogen atom, a carboxyl group, an amino group, and a variable R group (or side chain). n ¨ Differences in R groups produce the 20 different amino acids. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

The twenty different R groups may be as simple as a hydrogen atom (as in the amino acid glutamine) to a carbon skeleton with various functional groups attached. n The physical and chemical characteristics of the R group determine the unique characteristics of a particular amino acid. n Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n One group of amino acids has hydrophobic R groups. Fig. 5. 15 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• Another group of amino acids has polar R groups, making them hydrophilic. Fig. 5. 15 b Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n The last group of amino acids includes those with functional groups that are charged (ionized) at cellular p. H. ¨ Some R groups are bases, others are acids. Fig. 5. 15 c Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n Amino acids are joined together when a dehydration reaction removes a hydroxyl group from the carboxyl end of one amino acid and a hydrogen from the amino group of another. ¨ The resulting covalent bond is called a peptide bond. Fig. 5. 16 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n Repeating the process over and over creates a long polypeptide chain. ¨ At one end is an amino acid with a free amino group the (the N-terminus) and at the other is an amino acid with a free carboxyl group the (the C-terminus). The repeated sequence (N-C-C) is the polypeptide backbone. n Attached to the backbone are the various R groups. n Polypeptides range in size from a few monomers to thousands. n Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

2. A protein’s function depends on its specific conformation A functional proteins consists of one or more polypeptides that have been precisely twisted, folded, and coiled into a unique shape. n It is the order of amino acids that determines what the three-dimensional conformation will be. n Fig. 5. 17 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

A protein’s specific conformation determines its function. n In almost every case, the function depends on its ability to recognize and bind to some other molecule. n ¨ For example, antibodies bind to particular foreign substances that fit their binding sites. ¨ Enzyme recognize and bind to specific substrates, facilitating a chemical reaction. ¨ Neurotransmitters pass signals from one cell to another by binding to receptor sites on proteins in the membrane of the receiving cell. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

The folding of a protein from a chain of amino acids occurs spontaneously. n Three levels of structure: primary, secondary, and tertiary structure, are used to organize the folding within a single polypeptide. n Quarternary structure arises when two or more polypeptides join to form a protein. n Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

PRIMARY STRUCTURE n n n The primary structure is which amino acids are covalently bound together The numbers of amino acids vary (e. g. insulin 51, lysozyme 129, haemoglobin 574, gamma globulin 1250) The primary structure determines the folding of the polypeptide to give a functional protein Polar amino acids (acidic, basic and neutral) are hydrophilic and tend to be placed on the outside of the protein. Non-polar (hydrophobic) amino acids tend to be placed on the inside of the protein © 2007 Paul Billiet ODWS

Infinite variety The number of possible sequences is infinite An average protein has 300 amino acids, At each position there could be one of 20 different amino acids = 10390 possible combinations n Most are useless Natural selection picks out the best n © 2007 Paul Billiet ODWS

SECONDARY STRUCTURE The folding of the N-CC backbone of the polypeptide chain using weak hydrogen bonds © Text 2007 Paul Billiet ODWS © Science Student
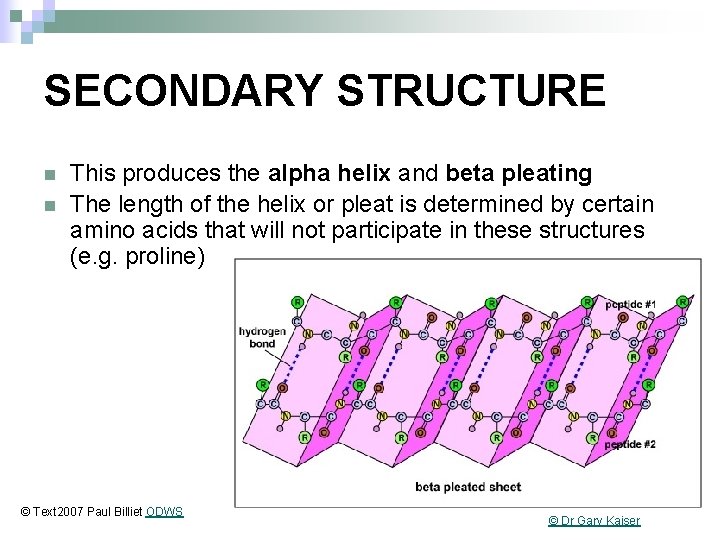
SECONDARY STRUCTURE n n This produces the alpha helix and beta pleating The length of the helix or pleat is determined by certain amino acids that will not participate in these structures (e. g. proline) © Text 2007 Paul Billiet ODWS © Dr Gary Kaiser

TERTIARY STRUCTURE The folding of the polypeptide into domains whose chemical properties are determined by the amino acids in the chain MIL 1 protein © 2007 Paul Billiet ODWS © Anne-Marie Ternes

TERTIARY STRUCTURE n n This folding is sometimes held together by strong covalent bonds (e. g. cysteine-cysteine disulphide bridge) Bending of the chain takes place at certain amino acids (e. g. proline) Hydrophobic amino acids tend to arrange themselves inside the molecule Hydrophilic amino acids arrange themselves on the outside © 2007 Paul Billiet ODWS

Chain B of Protein Kinase C © Max Planck Institute for Molecular Genetics

QUATERNARY STRUCTURE Some proteins are made of several polypeptide subunits (e. g. haemoglobin has four) Protein Kinase C © Max Planck Institute for Molecular Genetics © Text 2007 Paul Billiet ODWS

QUATERNARY STRUCTURE These subunits fit together to form the functional protein n Therefore, the sequence of the amino acids in the primary structure will influence the protein's structure at two, three or more levels n © 2007 Paul Billiet ODWS

Result Protein structure depends upon the amino acid sequence This, in turn, depends upon the sequence of bases in the gene

Let’s Discuss! n The diagram below shows a channel protein in a membrane. Which parts of the surface of the protein would be composed of polar amino acids? n HINT: The membrane is surrounded by an aqueous solution. A. I and II only B. II and III only C. III and IV only D. I and IV only n n

Quick Check – Hands Up! Which of the following reactions occurs when a dipeptide is formed from amino acids? n A. Hydrolysis n B. Denaturation n C. Condensation n D. Oxidation n

Quick Check – Hands Up! Which of the following represents the peptide linkage of a dipeptide? A. I n B. II n C. III n

PROTEIN FUNCTIONS Protein structure determines protein function n Denaturation or inhibition which may change protein structure will change its function n Coenzymes and cofactors in general may enhance the protein's structure n

Good Visual! http: //www. stolaf. edu/people/giannini/flash animat/proteins/protein%20 structure. swf n http: //www. stolaf. edu/people/giannini/flash animat/proteins/hydrophobic%20 force. swf n

Fibrous proteins Involved in structure: tendons ligaments blood clots (e. g. collagen and keratin) n Contractile proteins in movement: muscle, microtubules (cytoskelton, mitotic spindle, cilia, flagella) n

Globular Proteins Most proteins which move around (e. g. albumen, casein in milk) n Proteins with binding sites: enzymes, hemoglobin, immunoglobulin, membrane receptor sites n

Proteins classified by function n n n n CATALYTIC: enzymes STORAGE: ovalbumen (in eggs), casein (in milk), zein (in maize) TRANSPORT: haemoglobin COMMUNICATION: hormones (eg insulin) and neurotransmitters CONTRACTILE: actin, myosin, dynein (in microtubules) PROTECTIVE: Immunoglobulin, fibrinogen, blood clotting factors TOXINS: snake venom STRUCTURAL: cell membrane proteins, keratin (hair), collagen

Summary n Write a 3 -4 sentence summary about the topics of nucleic acids and proteins. ¨ Be sure to include a topic sentence as well as several details

Protein (review) n Examples of Proteins in the body: ¨ ¨ ¨ n Made up of just FIVE types of atoms: ¨ ¨ ¨ n Antibody (helps protect us from infection) Hormone (helps carry messages) Enzyme (Helps speed up reactions) Carbon Hydrogen Oxygen Sulfur Nitrogen Does the majority of the work in the cell. Examples include: ¨ ¨ Helps cells keep their shape Makes up muscle Speeds up chemical reactions Carries messages and material

protein n The subunits that make up proteins are called: ¨ AMINO ACIDS n There are 20 different kinds of amino acids in humans n The part that changes in different amino acids is the “R group” H Amino Acid N H AA AA AA R Group AA AA C COOH H AA AA

Protein n Long chains of amino acids make up a : ¨ Polypeptide One or more polypeptide together makes up a protein. n Each protein has FOUR different levels or structure. n ¨ Primary Structure ¨ Secondary Structure ¨ Tertiary Structure ¨ Quaternary Structure

Protein n The lowest level of protein structure is called its ¨ Primary Structure n You can tell a protein is in its primary structure if NO folding has occurred yet. n It will be just a sequence of amino acids ¨ AA AA AA Looks like beads on a string AA AA AA
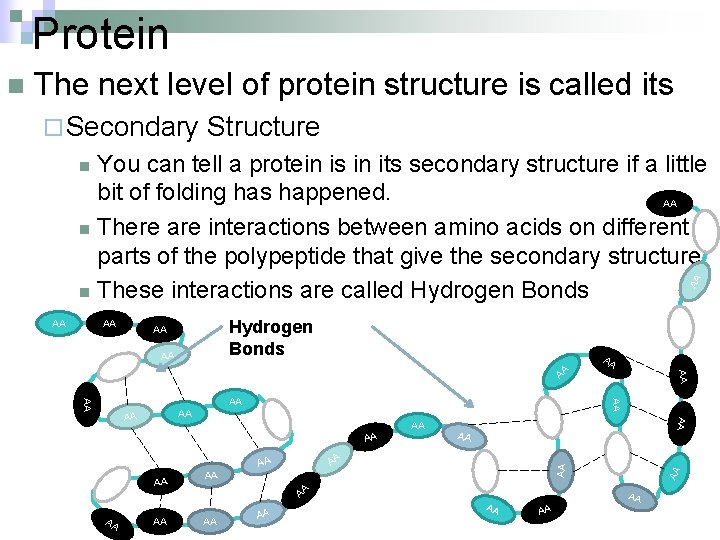
Protein The next level of protein structure is called its ¨ Secondary Structure You can tell a protein is in its secondary structure if a little bit of folding has happened. AA n There are interactions between amino acids on different parts of the polypeptide that give the secondary structure. n These interactions are called Hydrogen Bonds n AA AA Hydrogen Bonds AA AA AA AA AA AA AA AA AA n AA AA

AA AA A A A AA AA AA AA A A A A AA AA A A A AA AA A A A AA THIS SHAPE ALLOWS THE PROTEIN TO DO ITS JOB! ¨ A A A AA You can tell a protein is in its tertiary structure because it looks like a big ball of string with amino acids n The same interactions (hydrogen bonding) hold the large molecule in the right place or shape n REMEMBER!! A n A A AA ¨ Tertiary Structure A AA A A AA The third level of protein structure is called: A A AA n Protein A A

Protein The final level of protein structure is called: ¨ Quaternary Structure You can tell a protein is in its quaternary structure because it joins with other tertiary structured polypeptide to create a working protein. n The polypeptides interact with hydrogen bonding. The two or more polypeptides have A A to join together in a very A A A particular way. AA A A n REMEMBER!! A A A n A A A A A A A A A A A A A AA A A A A A A A AA A A A A A AA A A AA A A AA A A A A A A A A A A A A A AA A A A A A A A A A A A A THIS SHAPE ALLOWS THE PROTEIN TO DO ITS JOB! ¨ A A n A A

Protein n Proteins can serve one of two roles in the cell: ¨ Structure ¨ Function n Structural proteins- Provide support and structure to the cell and Organism ¨ (Ex. Fibers, Muscle…) n Functional Proteins- Do jobs for the cell ¨ (Ex. Antibodies, Hormones, Enzymes…)

Nucleic Acid (review) n Examples of Nucleic Acids in the body: ¨ DNA ¨ RNA n Made up of just FIVE types of atoms: ¨ Carbon ¨ Hydrogen ¨ Oxygen ¨ Nitrogen ¨ Phosphorous n Does THREE main jobs(functions) for the cell: ¨ Contains the instructions for how to build proteins ¨ Passes the instructions from parents to offspring ¨ Helps make proteins

CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Section D: Proteins - Many Structures, Many Functions Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n The primary structure of a protein is its unique sequence of amino acids. ¨ Lysozyme, an enzyme that attacks bacteria, consists on a polypeptide chain of 129 amino acids. ¨ The precise primary structure of a protein is determined by inherited genetic information. Fig. 5. 18 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Even a slight change in primary structure can affect a protein’s conformation and ability to function. n In individuals with sickle cell disease, abnormal hemoglobins, oxygen-carrying proteins, develop because of a single amino acid substitution. n ¨ These abnormal hemoglobins crystallize, deforming the red blood cells and leading to clogs in tiny blood vessels. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Fig. 5. 19 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n The secondary structure of a protein results from hydrogen bonds at regular intervals along the polypeptide backbone. ¨ Typical shapes that develop from secondary structure are coils (an alpha helix) or folds (beta pleated sheets). Fig. 5. 20 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n The structural properties of silk are due to beta pleated sheets. ¨ The presence of so many hydrogen bonds makes each silk fiber stronger than steel. Fig. 5. 21 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n Tertiary structure is determined by a variety of interactions among R groups and between R groups and the polypeptide backbone. ¨ These interactions include hydrogen bonds among polar and/or charged areas, ionic bonds between charged R groups, and hydrophobic interactions and van der Waals interactions among hydrophobic R Fig. 5. 22 groups. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n While these three interactions are relatively weak, disulfide bridges, strong covalent bonds that form between the sulfhydryl groups (SH) of cysteine monomers, stabilize the structure. Fig. 5. 22 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

n Quarternary structure results from the aggregation of two or more polypeptide subunits. ¨ Collagen is a fibrous protein of three polypeptides that are supercoiled like a rope. n This provides the structural strength for their role in connective tissue. ¨ Hemoglobin is a globular protein with two copies of two kinds of polypeptides. Fig. 5. 23 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Fig. 5. 24 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

A protein’s conformation can change in response to the physical and chemical conditions. n Alterations in p. H, salt concentration, temperature, or other factors can unravel or denature a protein. n ¨ These forces disrupt the hydrogen bonds, ionic bonds, and disulfide bridges that maintain the protein’s shape. n Some proteins can return to their functional shape after denaturation, but others cannot, especially in the crowded environment of the cell. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Fig. 5. 25 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Exit Ticket 1. The complex structure of proteins can be explained in terms of four levels of structure, primary, secondary, tertiary and quaternary. n (a) Primary structure involves the sequence of amino acids that are bonded together to form a polypeptide. State the name of the linkage that bonds the amino acids together. n (b) Beta pleated sheets are an example of secondary structure. State one other example. n (c) Tertiary structure in globular proteins involves the folding of polypeptides. State one type of bond that stabilizes the tertiary structure. 2. Which is not a primary function of protein molecules? n A. Hormones n B. Energy storage n C. Transport n D. Structure
- Slides: 64