Stoichiometry 101 Part C Solutions Solutions and Stoichiometry





- Slides: 22

Stoichiometry 101 Part C: Solutions

Solutions and Stoichiometry • Many times the reactants and/or products of chemical reactions are water solutions. • In these cases the concentration of the solution must be determined in order to determine amounts of reactants or products. • The concentration of a solution is a measure of the amount of solute that is dissolved in a given amount of solution.

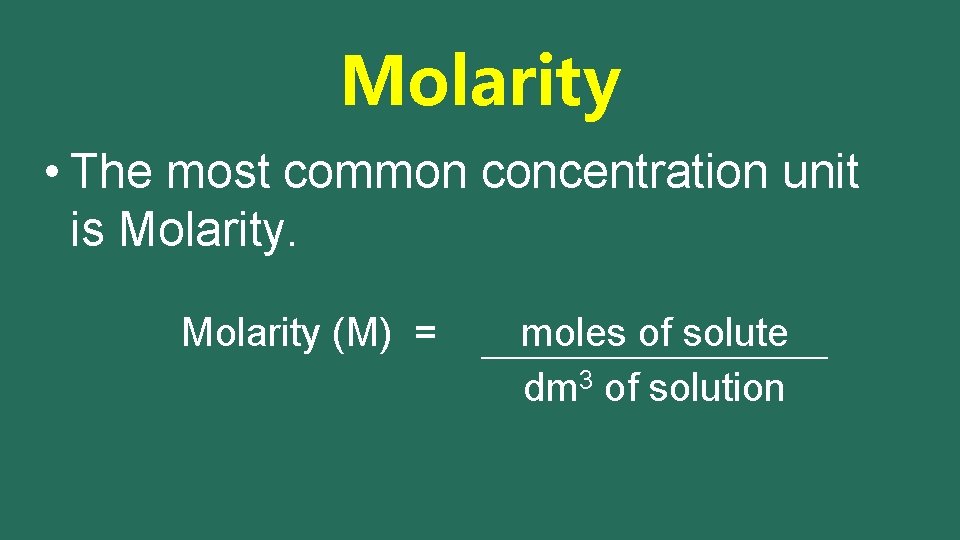
Molarity • The most common concentration unit is Molarity (M) = moles of solute dm 3 of solution

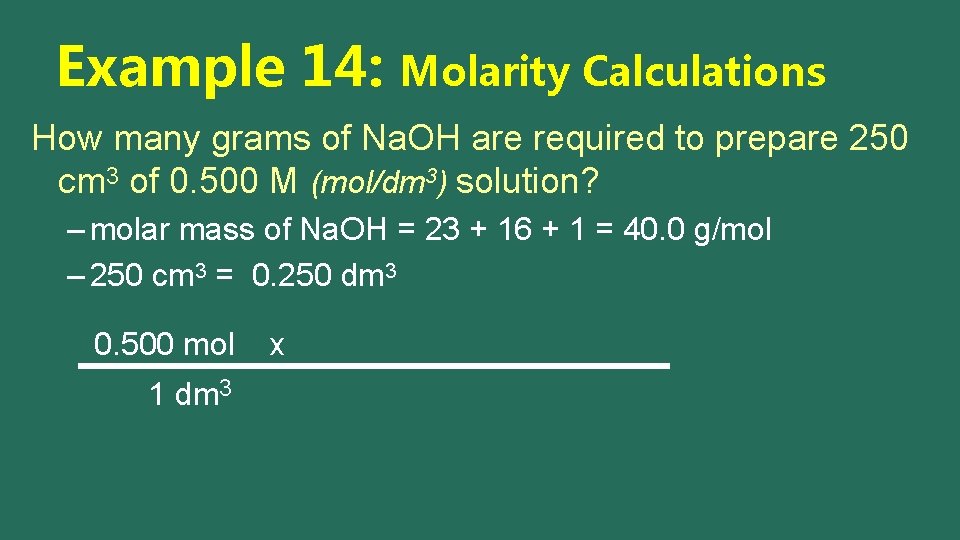
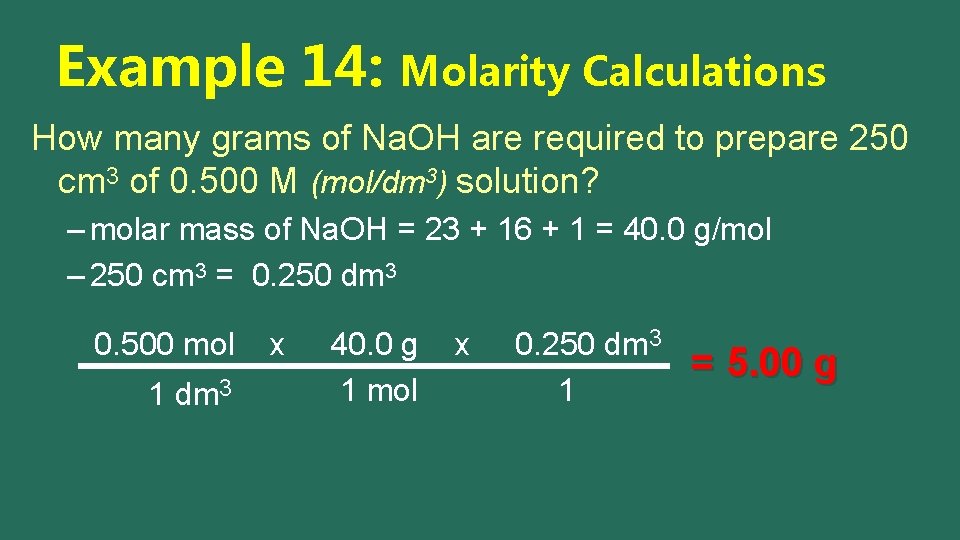
Example 14: Molarity Calculations How many grams of Na. OH are required to prepare 250 cm 3 of 0. 500 M (mol/dm 3) solution? – molar mass of Na. OH = 23 + 16 + 1 = 40. 0 g/mol – 250 cm 3 = 0. 250 dm 3 0. 500 mol 1 dm 3 x

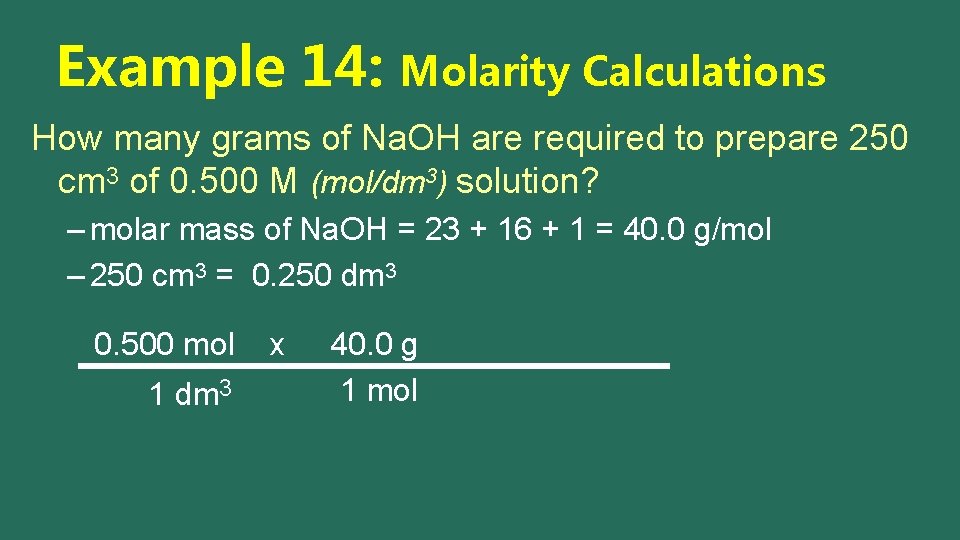
Example 14: Molarity Calculations How many grams of Na. OH are required to prepare 250 cm 3 of 0. 500 M (mol/dm 3) solution? – molar mass of Na. OH = 23 + 16 + 1 = 40. 0 g/mol – 250 cm 3 = 0. 250 dm 3 0. 500 mol 1 dm 3 x 40. 0 g 1 mol

Example 14: Molarity Calculations How many grams of Na. OH are required to prepare 250 cm 3 of 0. 500 M (mol/dm 3) solution? – molar mass of Na. OH = 23 + 16 + 1 = 40. 0 g/mol – 250 cm 3 = 0. 250 dm 3 0. 500 mol 1 dm 3 x 40. 0 g 1 mol x 0. 250 dm 3 1 = 5. 00 g

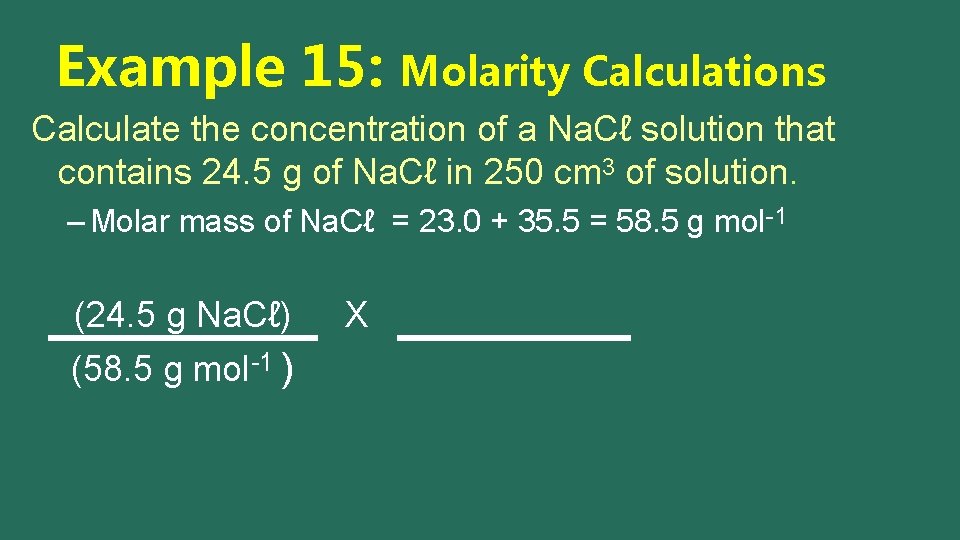
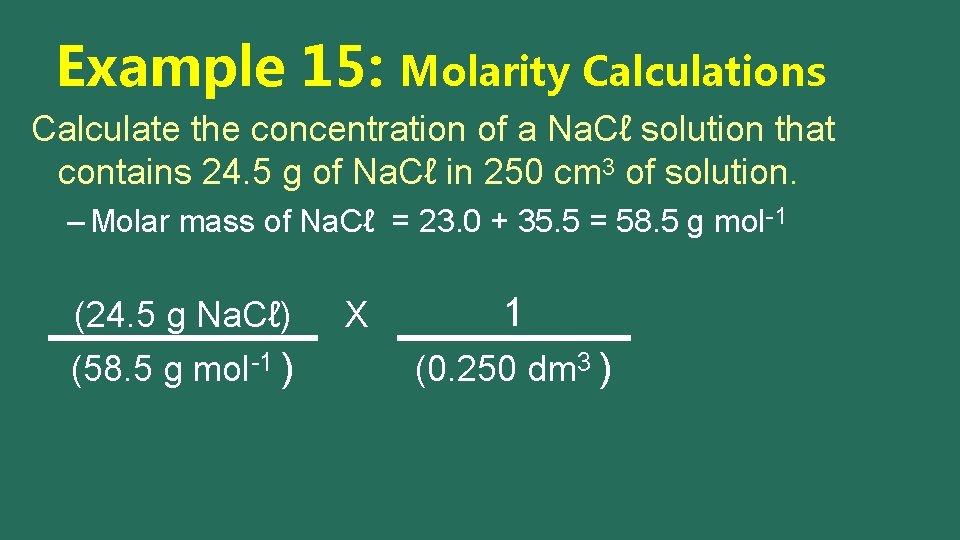
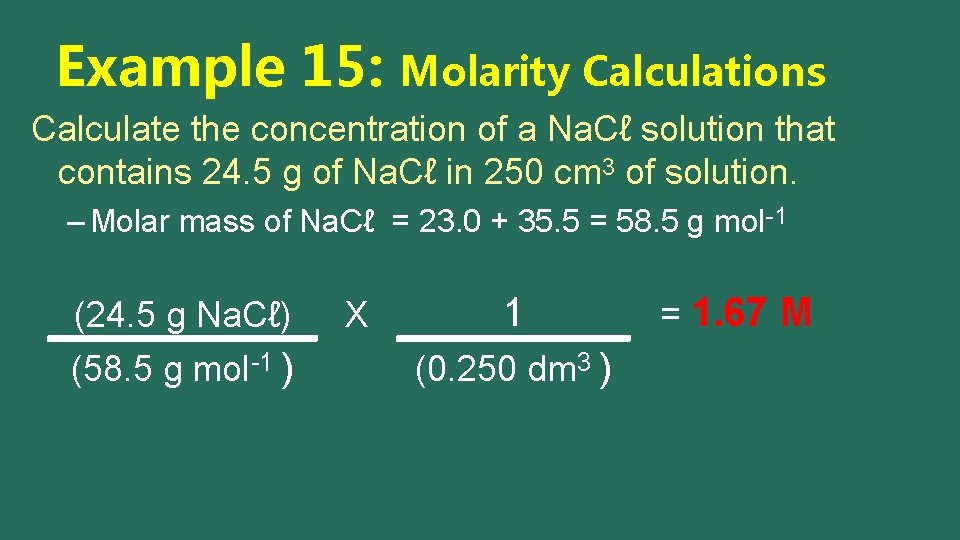
Example 15: Molarity Calculations Calculate the concentration of a Na. Cℓ solution that contains 24. 5 g of Na. Cℓ in 250 cm 3 of solution. – Molar mass of Na. Cℓ = 23. 0 + 35. 5 = 58. 5 g mol-1 (24. 5 g Na. Cℓ) (58. 5 g mol-1 ) X

Example 15: Molarity Calculations Calculate the concentration of a Na. Cℓ solution that contains 24. 5 g of Na. Cℓ in 250 cm 3 of solution. – Molar mass of Na. Cℓ = 23. 0 + 35. 5 = 58. 5 g mol-1 (24. 5 g Na. Cℓ) (58. 5 g mol-1 ) X 1 (0. 250 dm 3 )

Example 15: Molarity Calculations Calculate the concentration of a Na. Cℓ solution that contains 24. 5 g of Na. Cℓ in 250 cm 3 of solution. – Molar mass of Na. Cℓ = 23. 0 + 35. 5 = 58. 5 g mol-1 (24. 5 g Na. Cℓ) (58. 5 g mol-1 ) X 1 (0. 250 dm 3 ) = 1. 67 M

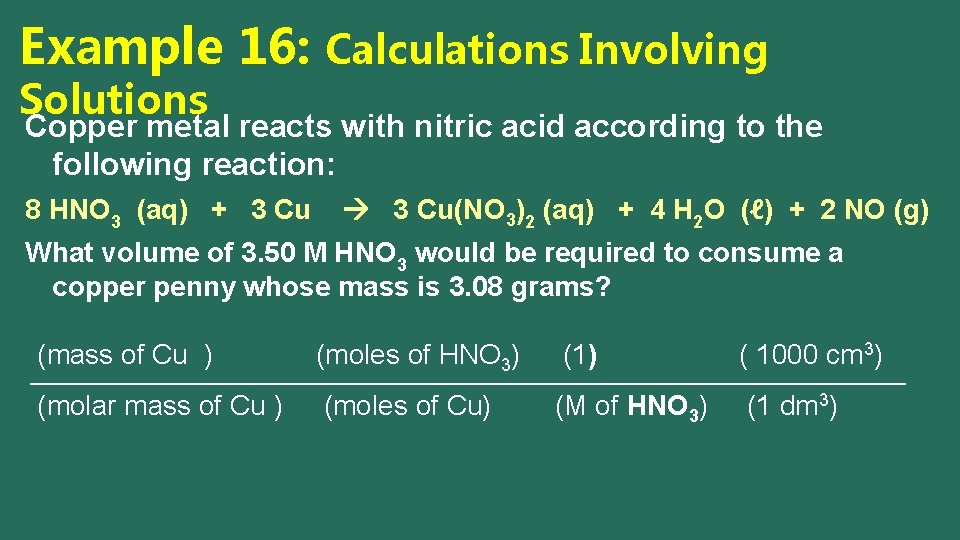
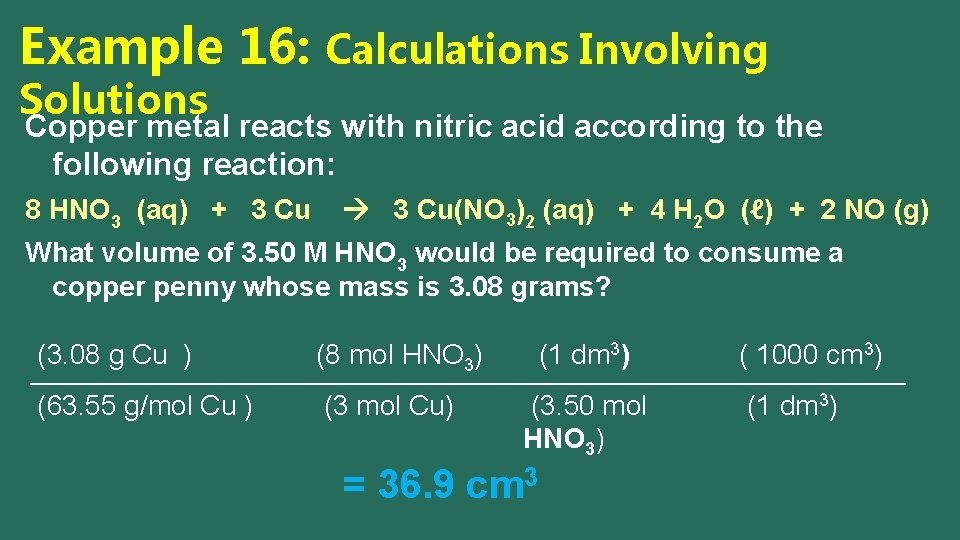
Example 16: Calculations Involving Solutions Copper metal reacts with nitric acid according to the following reaction: 8 HNO 3 (aq) + 3 Cu(NO 3)2 (aq) + 4 H 2 O (ℓ) + 2 NO (g) What volume of 3. 50 M HNO 3 would be required to consume a copper penny whose mass is 3. 08 grams? (mass of Cu ) (moles of HNO 3) (1) ( 1000 cm 3) (molar mass of Cu ) (moles of Cu) (M of HNO 3) (1 dm 3)

Example 16: Calculations Involving Solutions Copper metal reacts with nitric acid according to the following reaction: 8 HNO 3 (aq) + 3 Cu(NO 3)2 (aq) + 4 H 2 O (ℓ) + 2 NO (g) What volume of 3. 50 M HNO 3 would be required to consume a copper penny whose mass is 3. 08 grams? (3. 08 g Cu ) (8 mol HNO 3) (63. 55 g/mol Cu ) (3 mol Cu) (1 dm 3) (3. 50 mol HNO 3) = 36. 9 cm 3 ( 1000 cm 3) (1 dm 3)

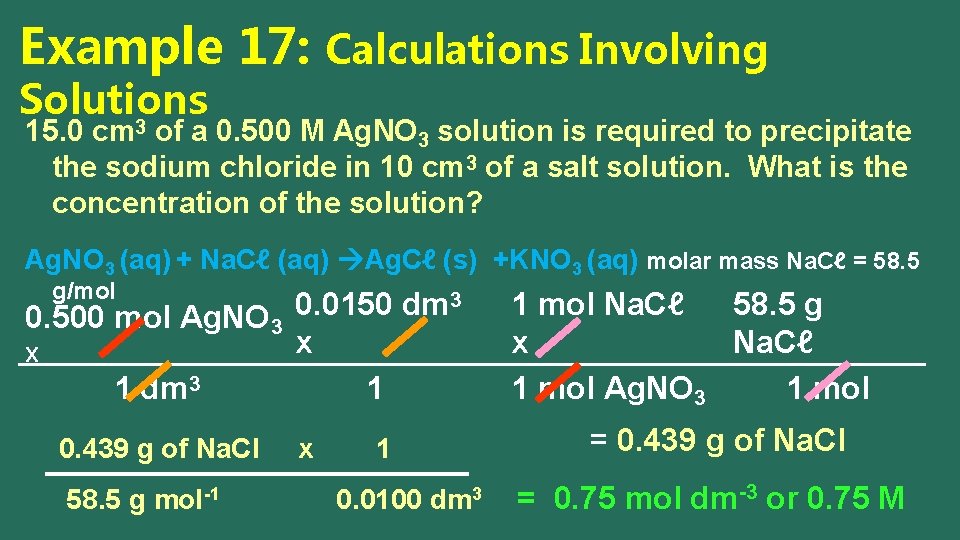
Example 17: Calculations Involving Solutions 3 15. 0 cm of a 0. 500 M Ag. NO 3 solution is required to precipitate the sodium chloride in 10 cm 3 of a salt solution. What is the concentration of the solution? Ag. NO 3 (aq) + Na. Cℓ (aq) Ag. Cℓ (s) +KNO 3 (aq) molar mass Na. Cℓ = 58. 5 g/mol 3 0. 0150 dm 0. 500 mol Ag. NO 3 x X 1 dm 3 1 0. 439 g of Na. Cl 58. 5 g mol-1 x 1 0. 0100 dm 3 1 mol Na. Cℓ 58. 5 g x Na. Cℓ 1 mol Ag. NO 3 1 mol = 0. 439 g of Na. Cl = 0. 75 mol dm-3 or 0. 75 M

Cookie Recipe Ingredients: • 1 cube butter • 1 cup canola oil • 2 cups white sugar • 1 egg • 1 teaspoon vanilla extract • 1/2 teaspoon salt • 1 teaspoon baking soda • 4 1/2 cups all-purpose flour • 1 cup oatmeal • 1 (12 ounce) package chocolate chips Makes 24 cookies In the kitchen there is: • 5 cubes butter • 8 cups canola oil • 8 cups white sugar • 12 eggs • 20 teaspoons vanilla extract • 1 pound salt • 40 teaspoons baking soda • 45 cups all-purpose flour • 30 cups oatmeal • 5 (12 ounce) packages chocolate chips • 5 pounds of dog biscuits How many cookies can I make without going to the store? WHY? ? ?

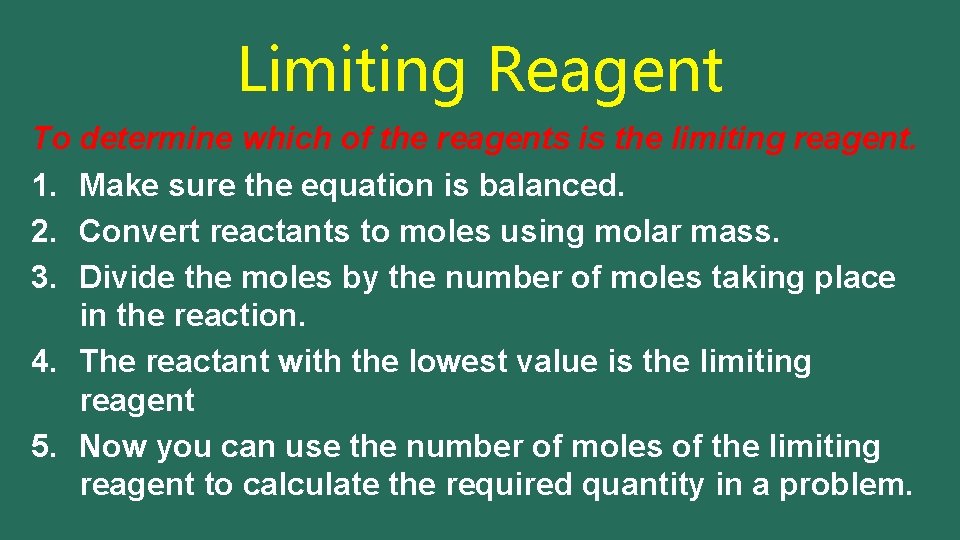
Limiting Reagent • The mole ratio determines how much of each reactant we need for the reaction. • Often we have an excess of one of the reactants. Then not all of that reactant will be used up. There will be some left over. • It is known as the excess reagent. • The other reactant will be used up and it will determine the amount of product we can form. • It is known as the limiting reagent. {cookie recipe}

Limiting Reagent To determine which of the reagents is the limiting reagent. 1. Make sure the equation is balanced. 2. Convert reactants to moles using molar mass. 3. Divide the moles by the number of moles taking place in the reaction. 4. The reactant with the lowest value is the limiting reagent 5. Now you can use the number of moles of the limiting reagent to calculate the required quantity in a problem.

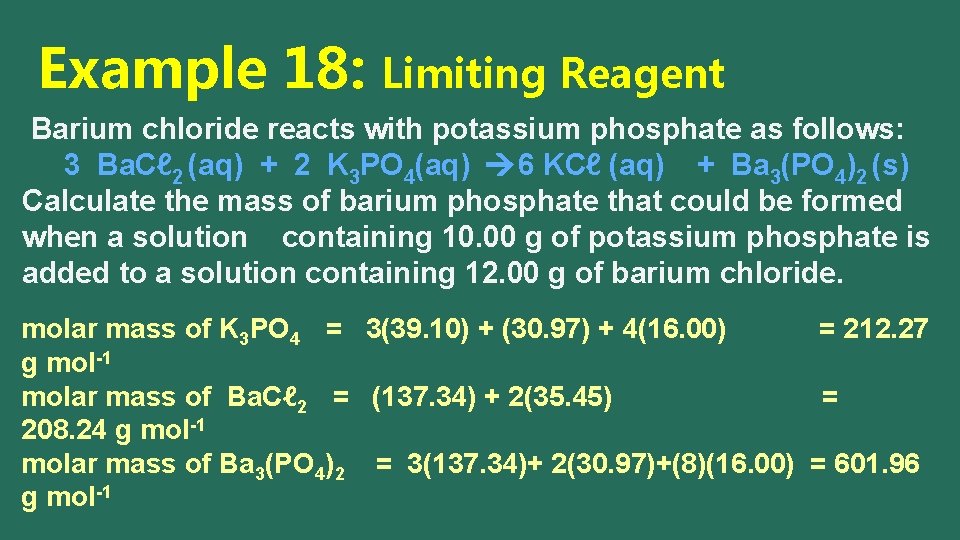
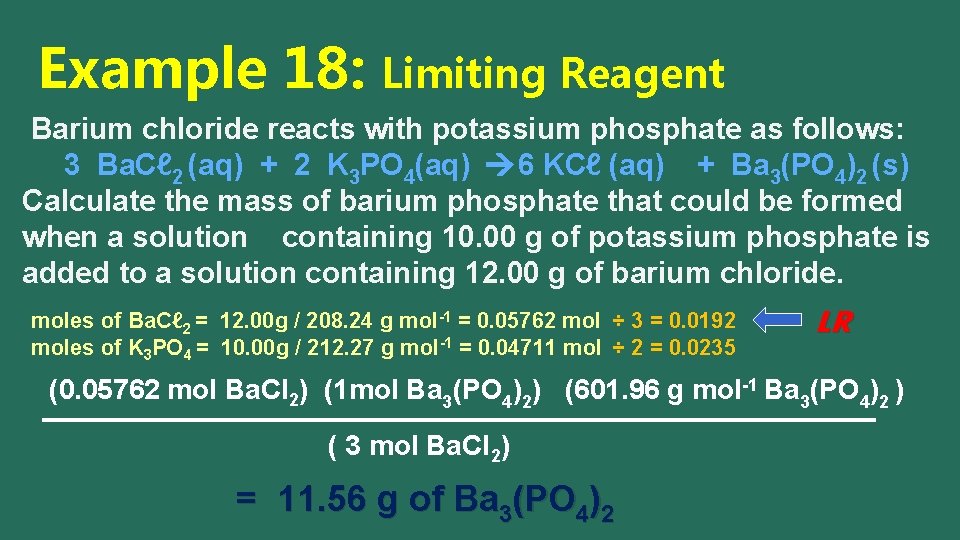
Example 18: Limiting Reagent Barium chloride reacts with potassium phosphate as follows: 3 Ba. Cℓ 2 (aq) + 2 K 3 PO 4(aq) 6 KCℓ (aq) + Ba 3(PO 4)2 (s) Calculate the mass of barium phosphate that could be formed when a solution containing 10. 00 g of potassium phosphate is added to a solution containing 12. 00 g of barium chloride. molar mass of K 3 PO 4 = 3(39. 10) + (30. 97) + 4(16. 00) = 212. 27 g mol-1 molar mass of Ba. Cℓ 2 = (137. 34) + 2(35. 45) = 208. 24 g mol-1 molar mass of Ba 3(PO 4)2 = 3(137. 34)+ 2(30. 97)+(8)(16. 00) = 601. 96 g mol-1

Example 18: Limiting Reagent Barium chloride reacts with potassium phosphate as follows: 3 Ba. Cℓ 2 (aq) + 2 K 3 PO 4(aq) 6 KCℓ (aq) + Ba 3(PO 4)2 (s) Calculate the mass of barium phosphate that could be formed when a solution containing 10. 00 g of potassium phosphate is added to a solution containing 12. 00 g of barium chloride. moles of Ba. Cℓ 2 = 12. 00 g / 208. 24 g mol-1 = 0. 05762 mol ÷ 3 = 0. 0192 moles of K 3 PO 4 = 10. 00 g / 212. 27 g mol-1 = 0. 04711 mol ÷ 2 = 0. 0235 LR (0. 05762 mol Ba. Cl 2) (1 mol Ba 3(PO 4)2) (601. 96 g mol-1 Ba 3(PO 4)2 ) ( 3 mol Ba. Cl 2) = 11. 56 g of Ba 3(PO 4)2

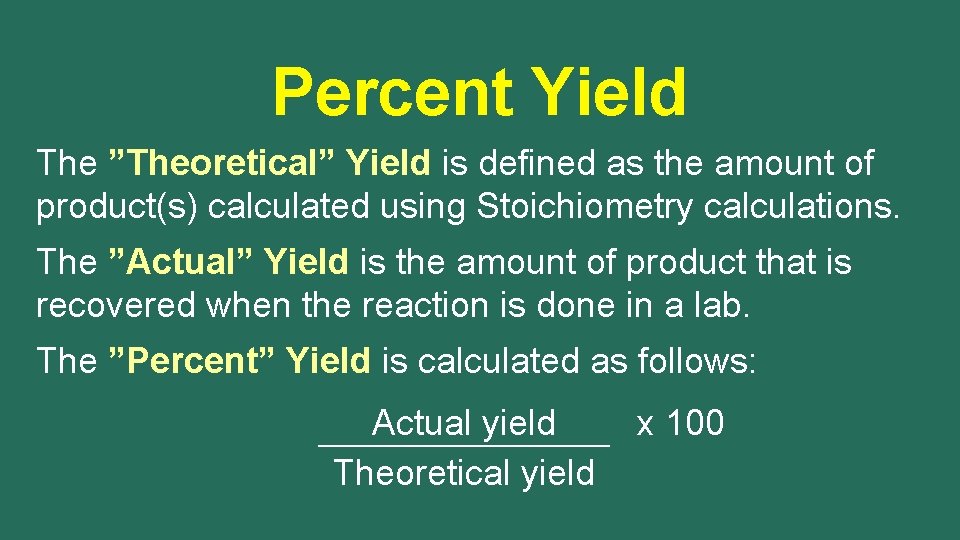
Percent Yield Stoichiometry allows us to calculate the amounts of reactants required or the amounts of products generated from a chemical reaction. Chemical reactions frequently do not proceed to completion, so the amount of product recovered is often less than would be predicted from calculations. In these situations it is helpful to calculate a percent yield.

Percent Yield The ”Theoretical” Yield is defined as the amount of product(s) calculated using Stoichiometry calculations. The ”Actual” Yield is the amount of product that is recovered when the reaction is done in a lab. The ”Percent” Yield is calculated as follows: Actual yield Theoretical yield x 100

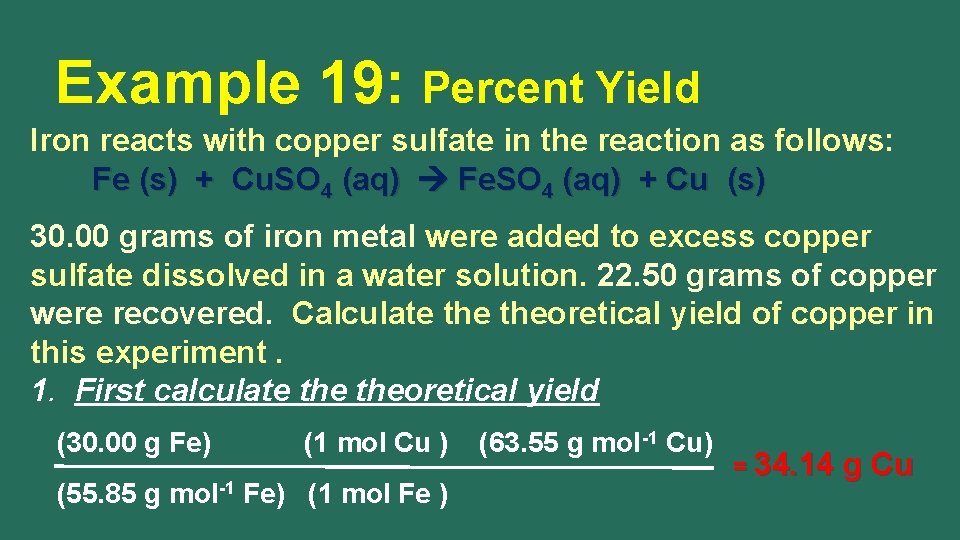
Example 19: Percent Yield Iron reacts with copper sulfate in the reaction as follows: Fe (s) + Cu. SO 4 (aq) Fe. SO 4 (aq) + Cu (s) 30. 00 grams of iron metal were added to excess copper sulfate dissolved in a water solution. 22. 50 grams of copper were recovered. Calculate theoretical yield of copper in this experiment. 1. First calculate theoretical yield (30. 00 g Fe) (1 mol Cu ) (55. 85 g mol-1 Fe) (1 mol Fe ) (63. 55 g mol-1 Cu) = 34. 14 g Cu

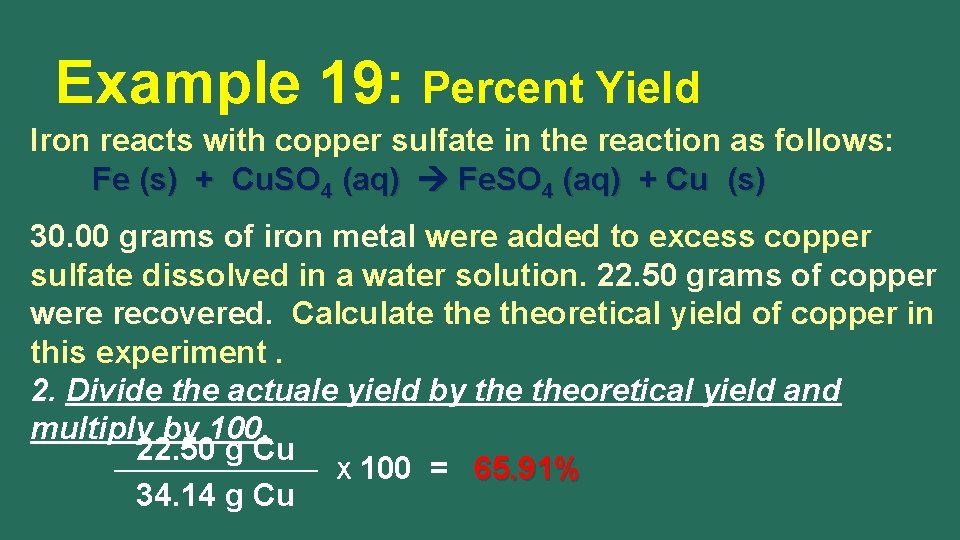
Example 19: Percent Yield Iron reacts with copper sulfate in the reaction as follows: Fe (s) + Cu. SO 4 (aq) Fe. SO 4 (aq) + Cu (s) 30. 00 grams of iron metal were added to excess copper sulfate dissolved in a water solution. 22. 50 grams of copper were recovered. Calculate theoretical yield of copper in this experiment. 2. Divide the actuale yield by theoretical yield and multiply by 100. 22. 50 g Cu X 100 = 65. 91% 34. 14 g Cu
