Solutions I What is a solution A solution

















- Slides: 55

Solutions

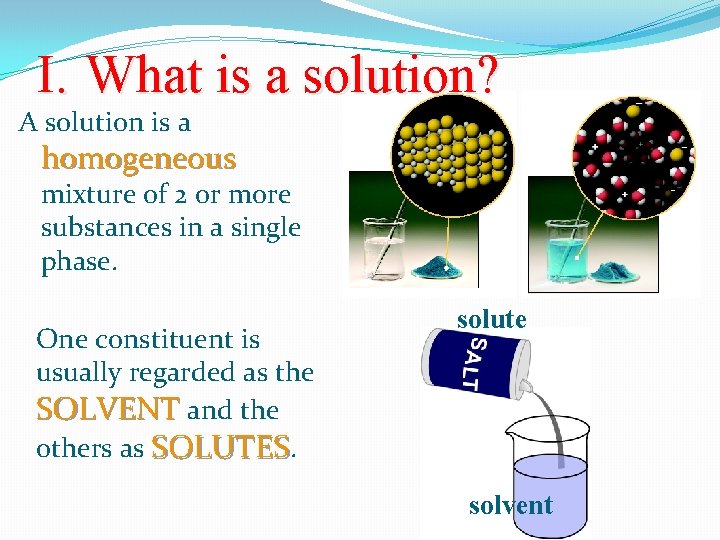
I. What is a solution? A solution is a homogeneous mixture of 2 or more substances in a single phase. One constituent is usually regarded as the SOLVENT and the others as SOLUTES. solute solvent

Parts of a Solution �SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) �SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) �Solute + Solvent = Solution Solvent Solute

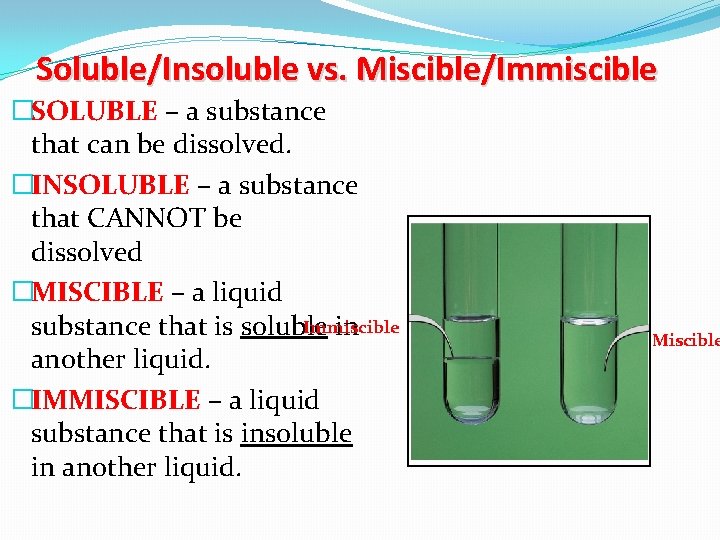
Soluble/Insoluble vs. Miscible/Immiscible �SOLUBLE – a substance that can be dissolved. �INSOLUBLE – a substance that CANNOT be dissolved �MISCIBLE – a liquid Immiscible substance that is soluble in another liquid. �IMMISCIBLE – a liquid substance that is insoluble in another liquid. Miscible

Types of Solutions 1. Gaseous solutions 2. Liquid solutions 3. Solid solutions--alloys

Types of Solutions Examples Gas in a Liquid in a Liquid Solids in Solids Non-Examples salad soil Air Soda Gasoline Sea Water Brass water

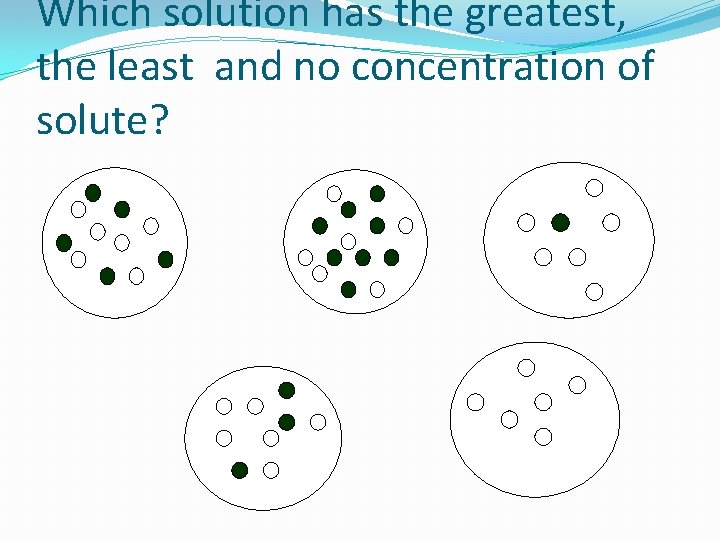
Which solution has the greatest, the least and no concentration of solute?

Nature of Solutes in Solutions l Spread evenly throughout the solution l Cannot be separated by filtration l Can be separated by evaporation l Not visible, solution appears transparent l May give a color to the solution 8

Water is the universal solvent �because more substances dissolve in water than in any other chemical. �This has to do with the polarity of each water molecule.

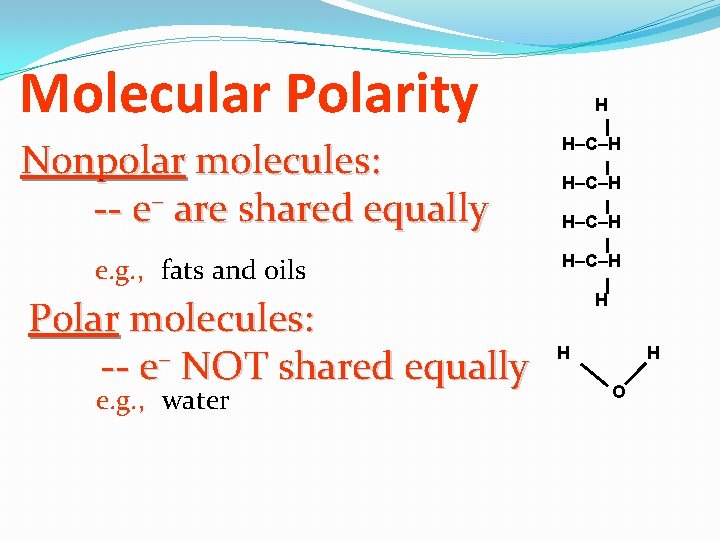
Molecular Polarity Nonpolar molecules: -- e– are shared equally e. g. , fats and oils Polar molecules: -- e– NOT shared equally e. g. , water H H–C–H H O

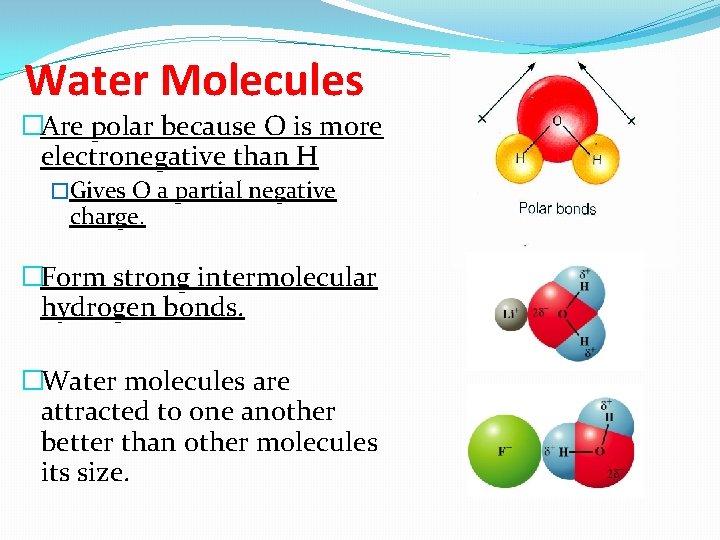
Water Molecules �Are polar because O is more electronegative than H �Gives O a partial negative charge. �Form strong intermolecular hydrogen bonds. �Water molecules are attracted to one another better than other molecules its size.

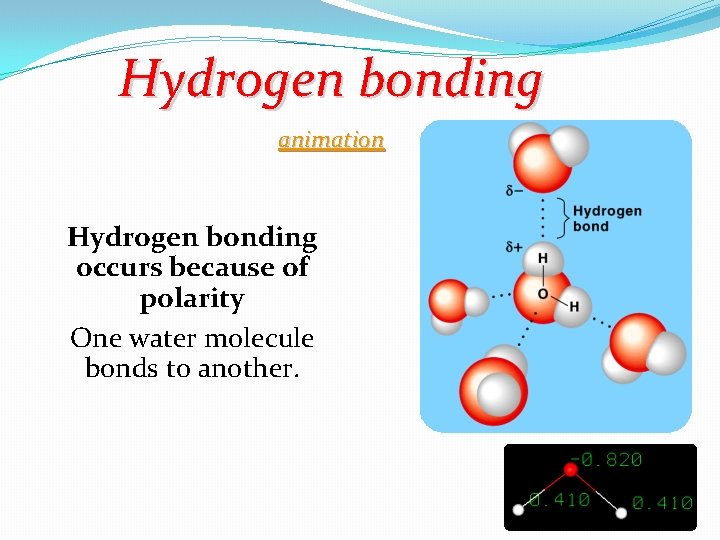
Hydrogen bonding animation Hydrogen bonding occurs because of polarity One water molecule bonds to another. 12

What is, or is not, soluble in H 20? Like Dissolves Like �“Polar solvents dissolve ionic compounds and polar molecules �Water is polar therefore it can dissolve �Na. Cl �Copper (II) sulfate �Na. OH �Nonpolar solvents dissolve nonpolar compounds �Oil is nonpolar, which is why oil and water separate

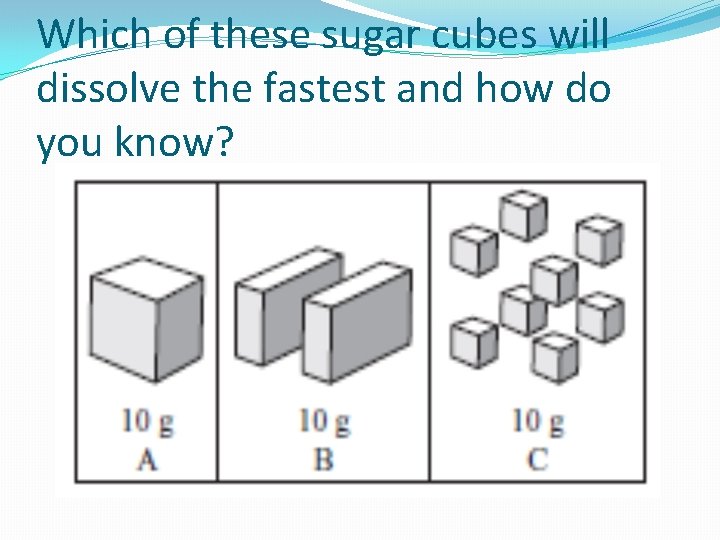
Which of these sugar cubes will dissolve the fastest and how do you know?

Animations of the Solvation (Animation of a Solute Dissolving) While you watch each video clip, record your observations on your notes. Animation of Salt Dissolving in Water Animation of Sugar Dissolving in Water

Aqueous Solutions How do we know ions are present in aqueous solutions? The solutions can electricity conduct They are called ELECTROLYTES Examples: HCl, Mg. Cl 2, and Na. Cl

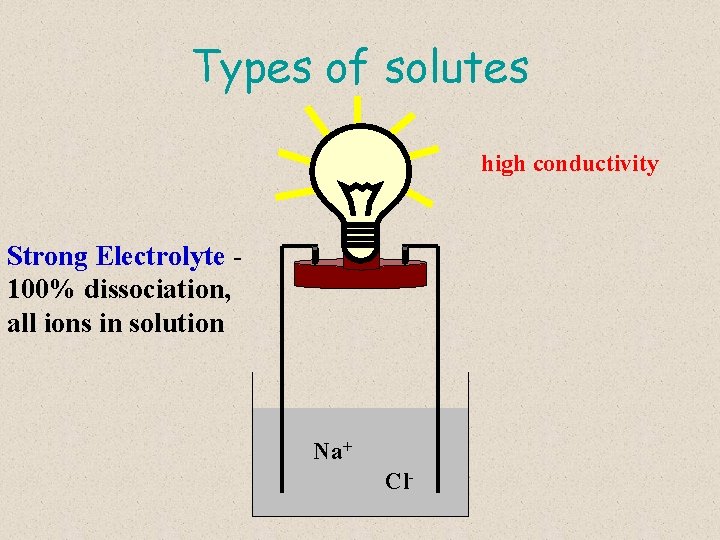
Types of solutes high conductivity Strong Electrolyte 100% dissociation, all ions in solution Na+ Cl-

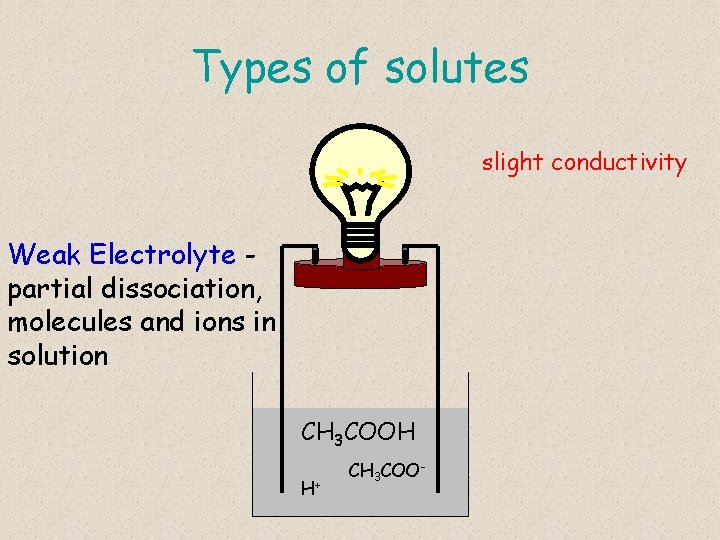
Types of solutes slight conductivity Weak Electrolyte partial dissociation, molecules and ions in solution CH 3 COOH H+ CH 3 COO-

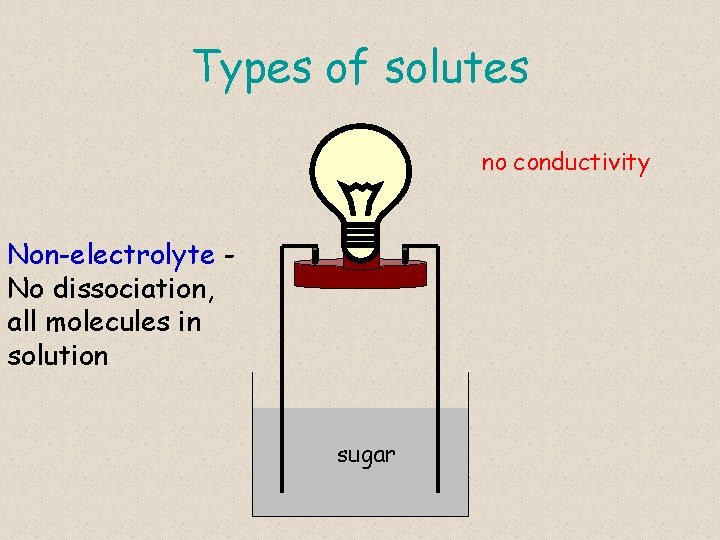
Types of solutes no conductivity Non-electrolyte No dissociation, all molecules in solution sugar

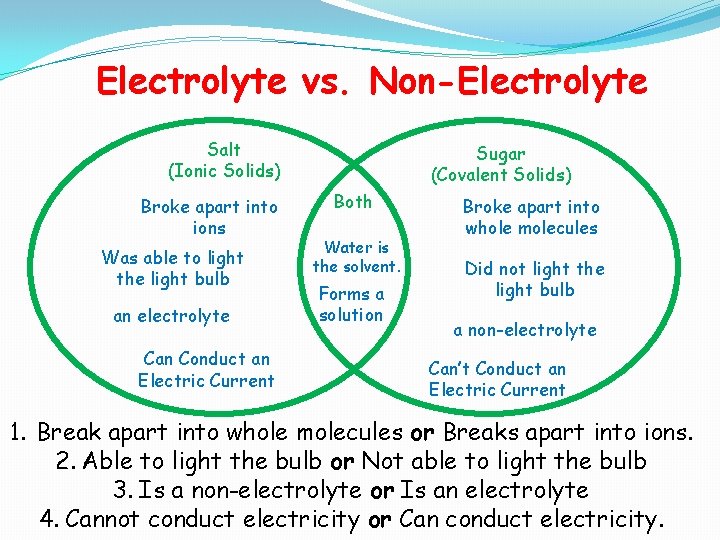
Electrolyte vs. Non-Electrolyte Salt (Ionic Solids) Broke apart into ions Was able to light the light bulb an electrolyte Can Conduct an Electric Current Sugar (Covalent Solids) Both Water is the solvent. Forms a solution Broke apart into whole molecules Did not light the light bulb a non-electrolyte Can’t Conduct an Electric Current 1. Break apart into whole molecules or Breaks apart into ions. 2. Able to light the bulb or Not able to light the bulb 3. Is a non-electrolyte or Is an electrolyte 4. Cannot conduct electricity or Can conduct electricity.

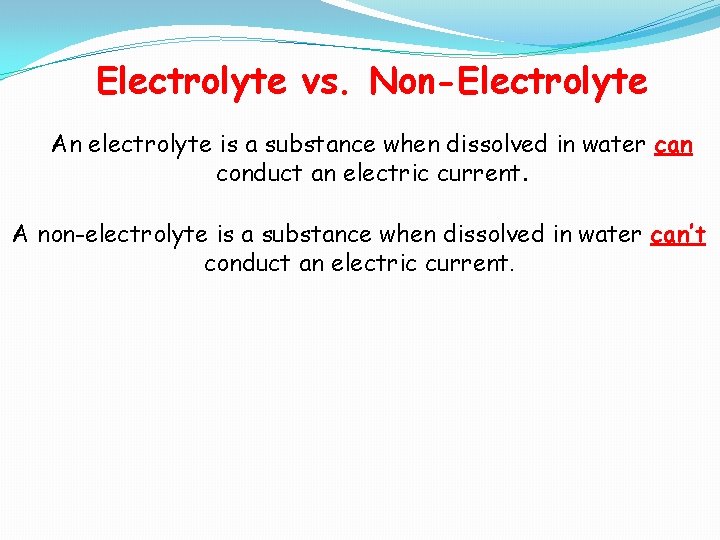
Electrolyte vs. Non-Electrolyte An electrolyte is a substance when dissolved in water can conduct an electric current. A non-electrolyte is a substance when dissolved in water can’t conduct an electric current.

Rate of Solution = How Fast Exploration 1) To an empty 250 m. L beaker, add approximately 100 m. L of warm-hot water from a hot plate. 2) To an empty 250 m. L beaker, add approximately 100 m. L of ice water. Be sure to leave the ice behind! 3) Add a sugar cube to each of the 250 m. L beakers. Observe what happens. 4) Record your observations on your notes. 5) List other ways that you believe that you could make a solute dissolve more quickly.

Electrolytes in the Body Carry messages to and from the brain as electrical signals Maintain cellular function with the correct concentrations electrolytes Make your own 50 -70 g sugar One liter of warm water Pinch of salt 200 ml of sugar free fruit squash Mix, cool and drink

Rate of Solution What are ways that you make a solute dissolve faster in water? 1) Increase the temperature. 2) Crush or use smaller size solute particles. 3) Stir the solutions.

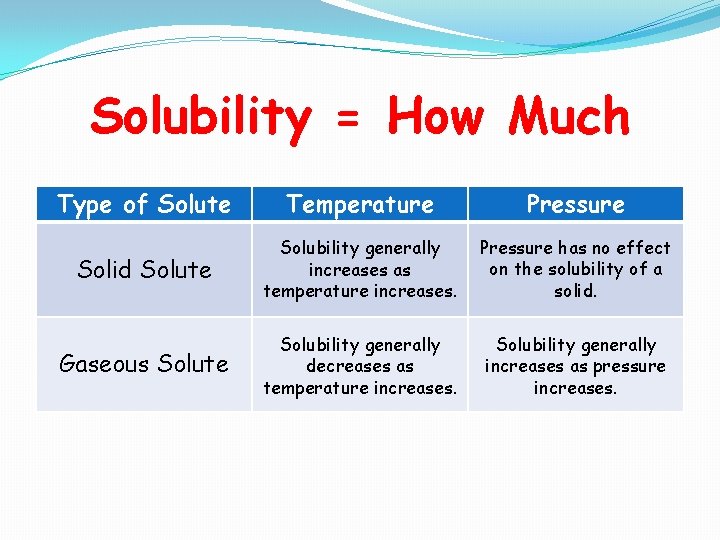
Solubility = How Much Type of Solute Temperature Pressure Solid Solute Solubility generally increases as temperature increases. Pressure has no effect on the solubility of a solid. Gaseous Solute Solubility generally decreases as temperature increases. Solubility generally increases as pressure increases.

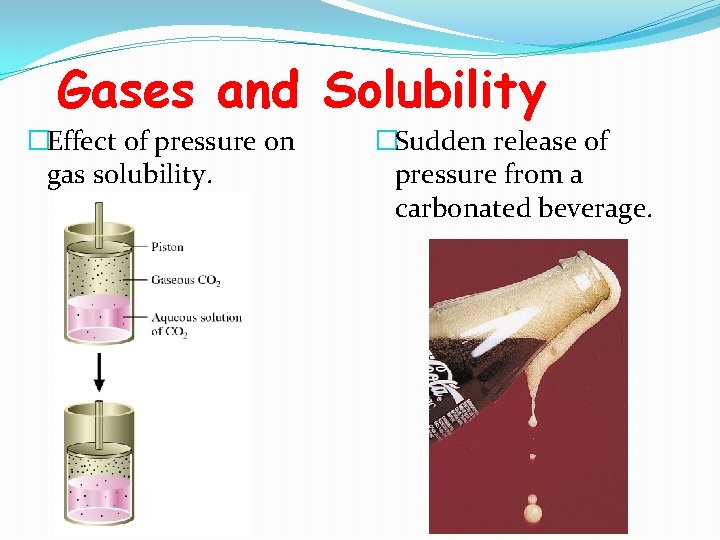
Gases and Solubility �Effect of pressure on gas solubility. �Sudden release of pressure from a carbonated beverage.

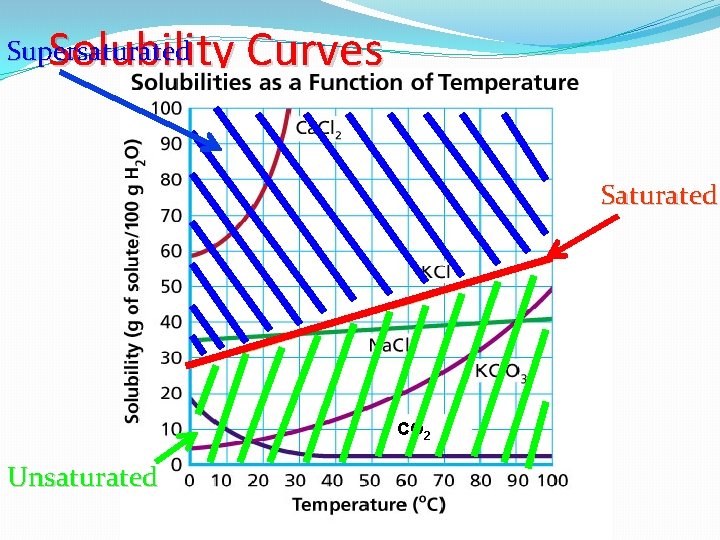
Types of Solutions There are three ways to classify a solution. 1) Unsaturated Solutions 2) Saturated Solutions 3) Supersaturated Solutions

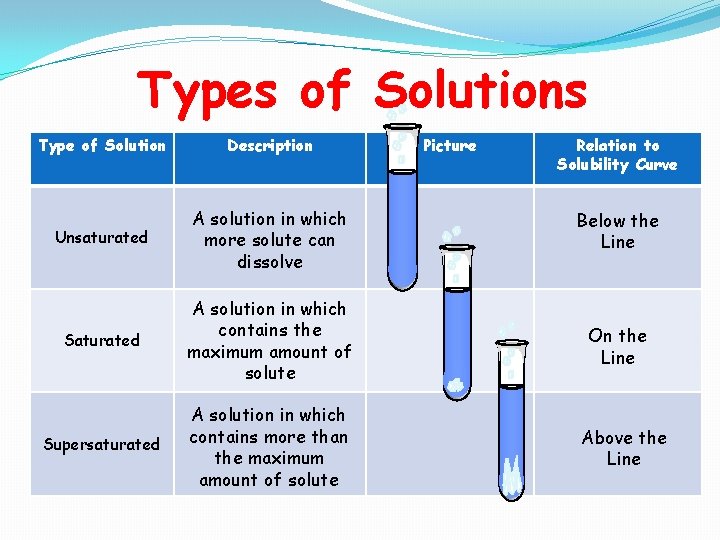
Types of Solutions Type of Solution Description Picture Relation to Solubility Curve Unsaturated A solution in which more solute can dissolve Below the Line Saturated A solution in which contains the maximum amount of solute On the Line Supersaturated A solution in which contains more than the maximum amount of solute Above the Line

Supersaturated �The solution is holding more solute than it should be able to. This is achieved by heating the solution and then cooling it slowly. �Examples: rock candy, southern style sweet tea, chemical heat packs �Supersaturated solutions are unstable. The supersaturation is only temporary

Supersaturated

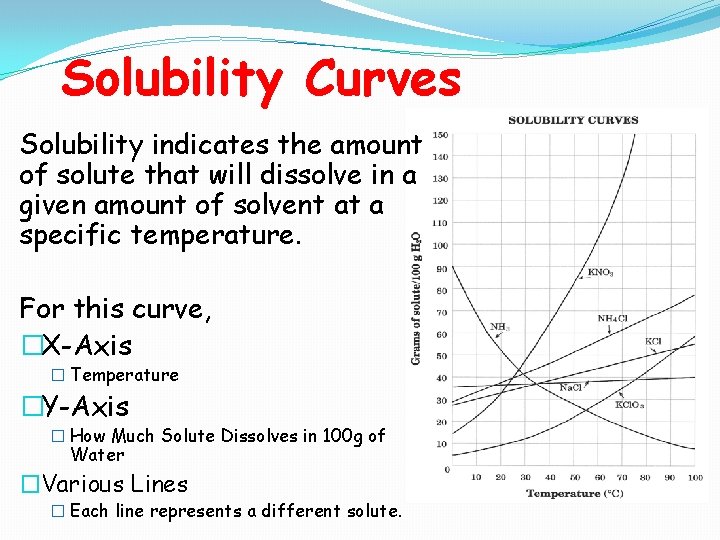
Solubility Curves Solubility indicates the amount of solute that will dissolve in a given amount of solvent at a specific temperature. For this curve, �X-Axis � Temperature �Y-Axis � How Much Solute Dissolves in 100 g of Water �Various Lines � Each line represents a different solute.

Solubility Curves Supersaturated Saturated CO 2 Unsaturated

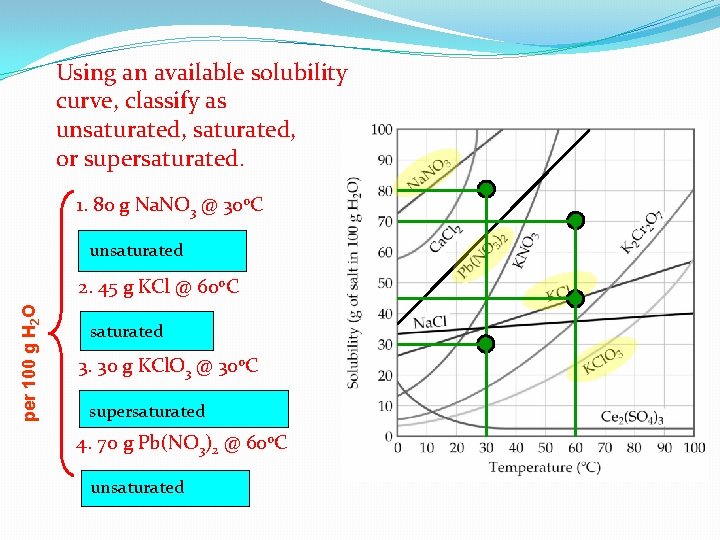
Using an available solubility curve, classify as unsaturated, or supersaturated. 1. 80 g Na. NO 3 @ 30 o. C unsaturated per 100 g H 2 O 2. 45 g KCl @ 60 o. C saturated 3. 30 g KCl. O 3 @ 30 o. C supersaturated 4. 70 g Pb(NO 3)2 @ 60 o. C unsaturated

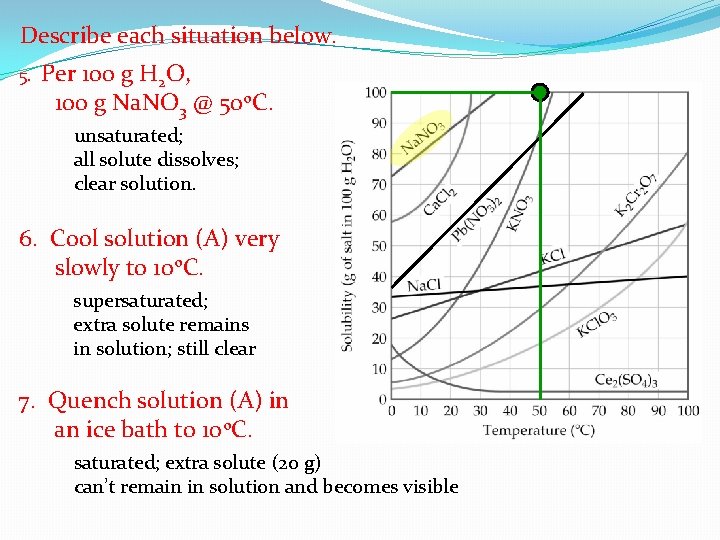
Describe each situation below. 5. Per 100 g H 2 O, 100 g Na. NO 3 @ 50 o. C. unsaturated; all solute dissolves; clear solution. 6. Cool solution (A) very slowly to 10 o. C. supersaturated; extra solute remains in solution; still clear 7. Quench solution (A) in an ice bath to 10 o. C. saturated; extra solute (20 g) can’t remain in solution and becomes visible

How to use a solubility graph? A. IDENTIFYING A SUBSTANCE ( given the solubility in g/100 cm 3 of water and the temperature) • Look for the intersection of the solubility and temperature.

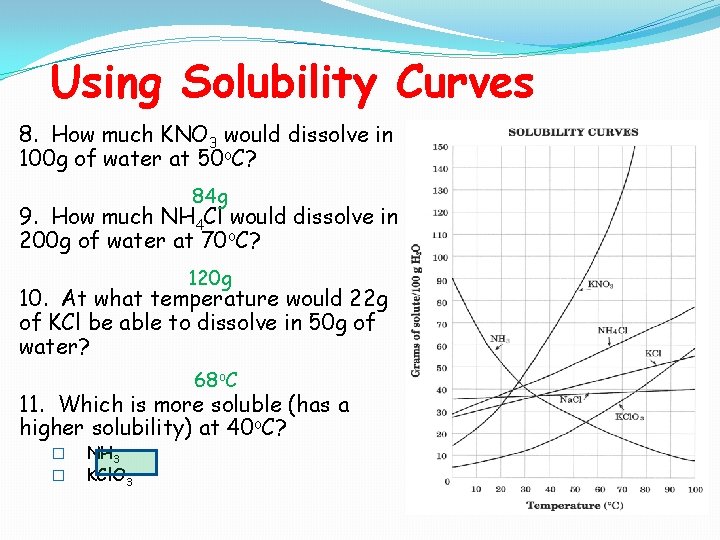
Using Solubility Curves 8. How much KNO 3 would dissolve in 100 g of water at 50 o. C? 84 g 9. How much NH 4 Cl would dissolve in 200 g of water at 70 o. C? 120 g 10. At what temperature would 22 g of KCl be able to dissolve in 50 g of water? 68 o. C 11. Which is more soluble (has a higher solubility) at 40 o. C? � � NH 3 KCl. O 3

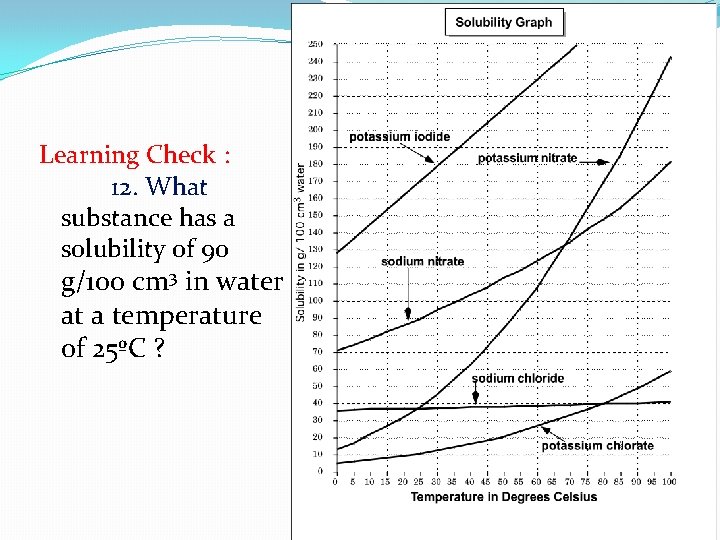
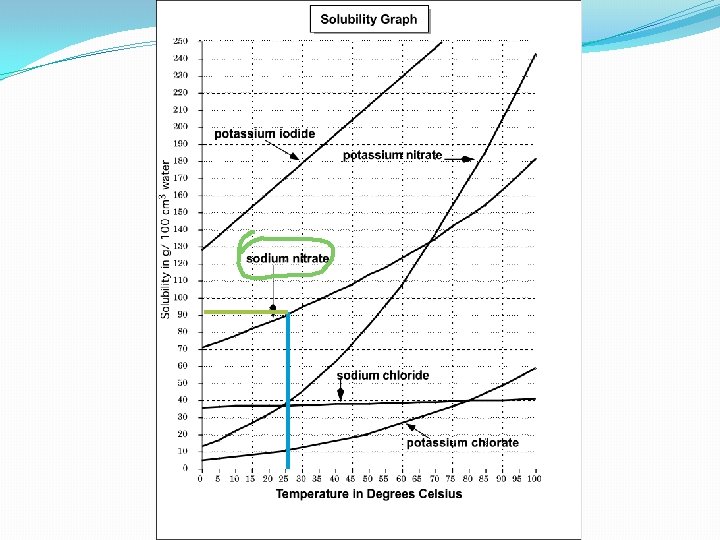
Learning Check : 12. What substance has a solubility of 90 g/100 cm 3 in water at a temperature of 25ºC ?


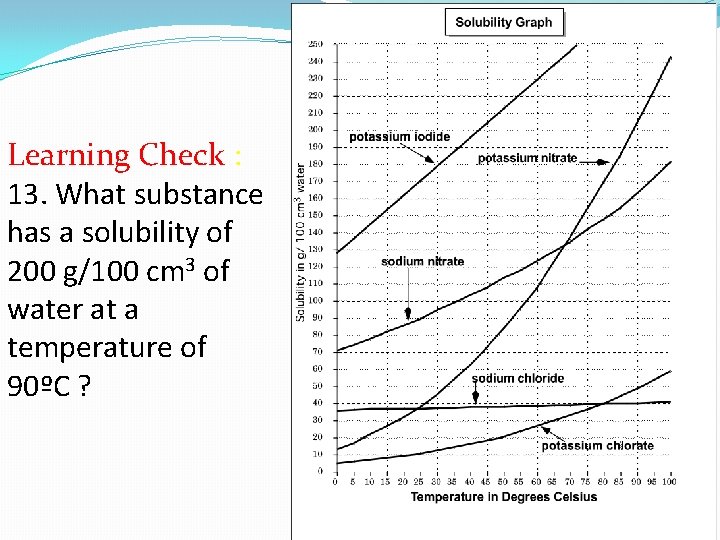
Learning Check : 13. What substance has a solubility of 200 g/100 cm 3 of water at a temperature of 90ºC ?


Look for the temperature or solubility • Locate the solubility curve needed and see for a given temperature, which solubility it lines up with and visa versa.

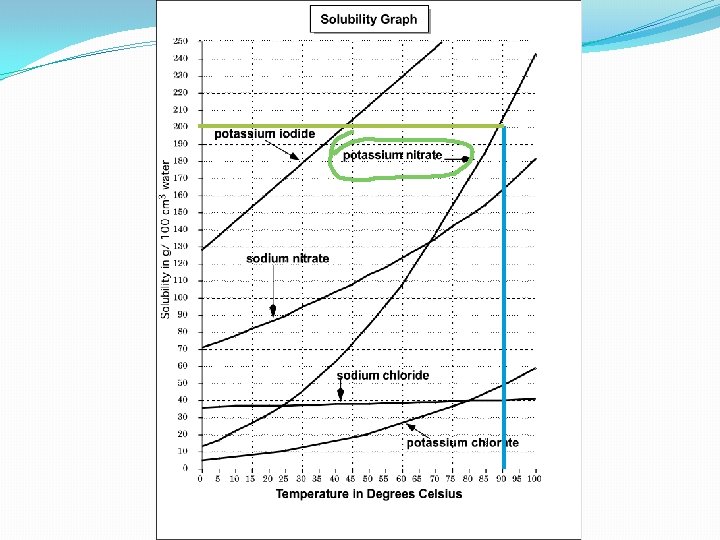
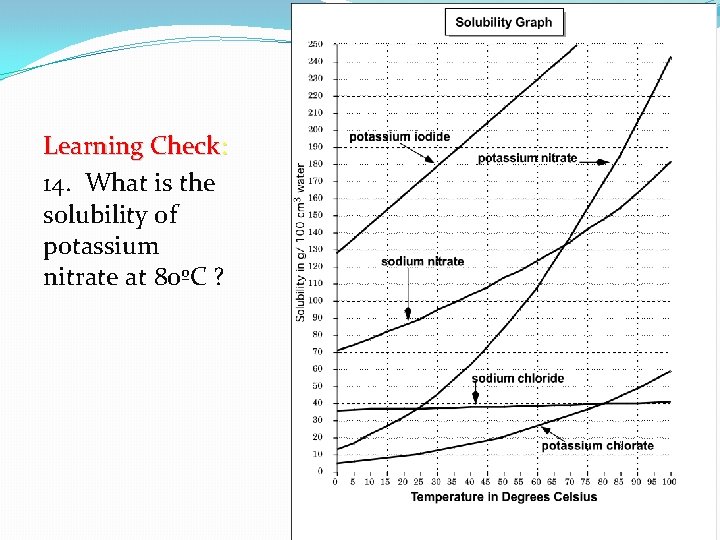
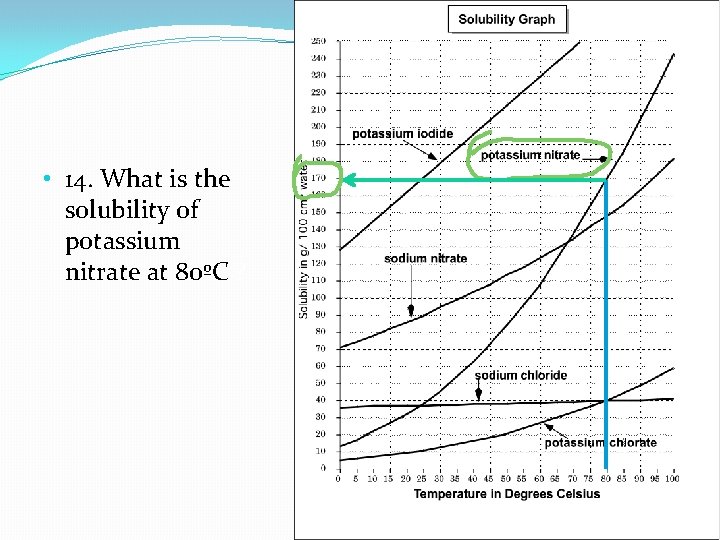
Learning Check: 14. What is the solubility of potassium nitrate at 80ºC ?

• 14. What is the solubility of potassium nitrate at 80ºC ?

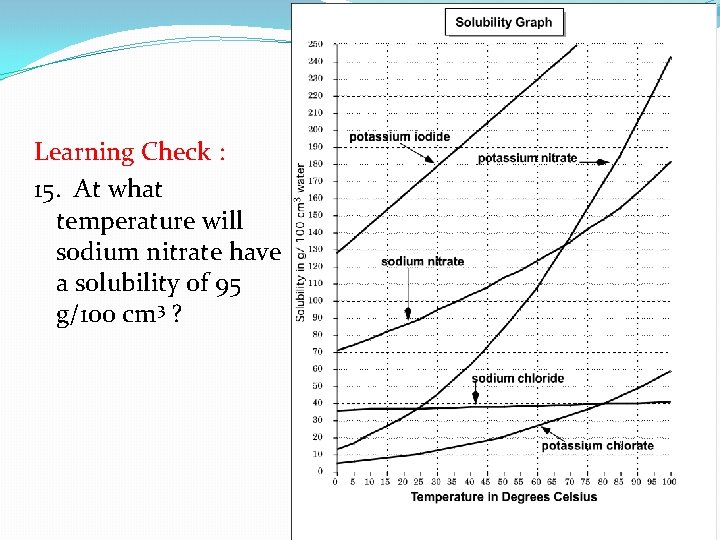
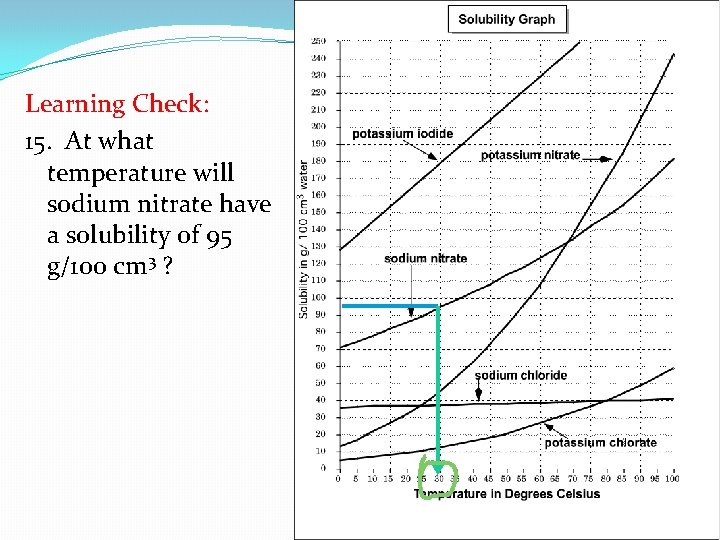
Learning Check : 15. At what temperature will sodium nitrate have a solubility of 95 g/100 cm 3 ?

Learning Check: 15. At what temperature will sodium nitrate have a solubility of 95 g/100 cm 3 ?

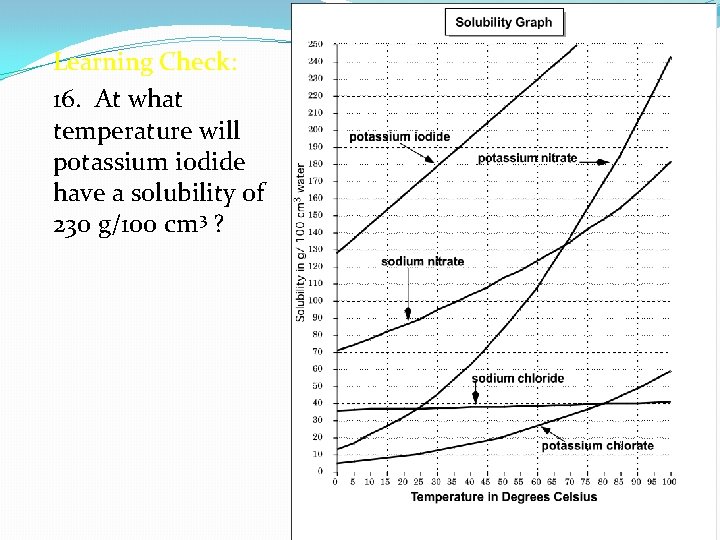
Learning Check: 16. At what temperature will potassium iodide have a solubility of 230 g/100 cm 3 ?

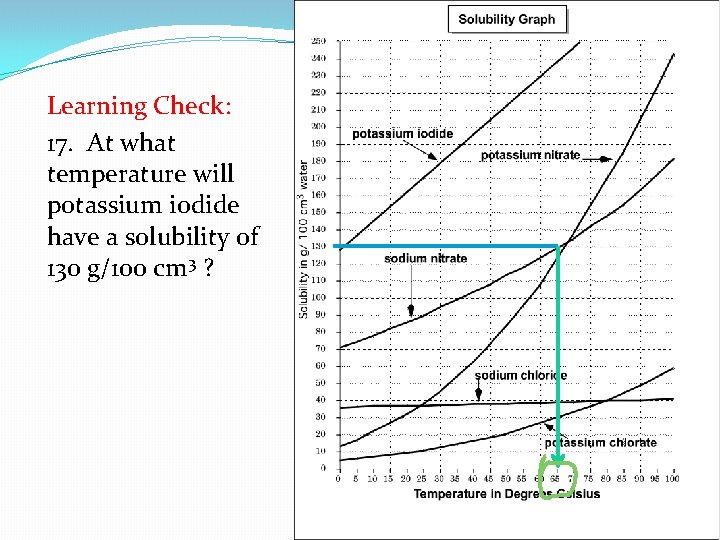
Learning Check: 17. At what temperature will potassium iodide have a solubility of 130 g/100 cm 3 ?

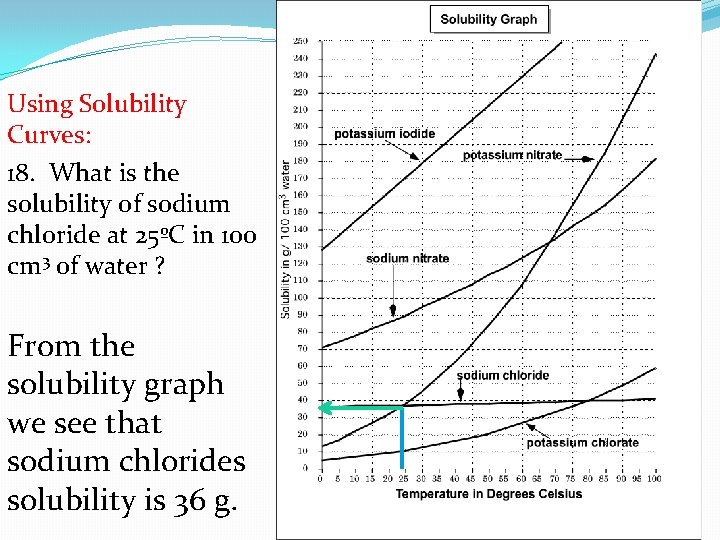
Using Solubility Curves: 18. What is the solubility of sodium chloride at 25ºC in 100 cm 3 of water ? From the solubility graph we see that sodium chlorides solubility is 36 g.

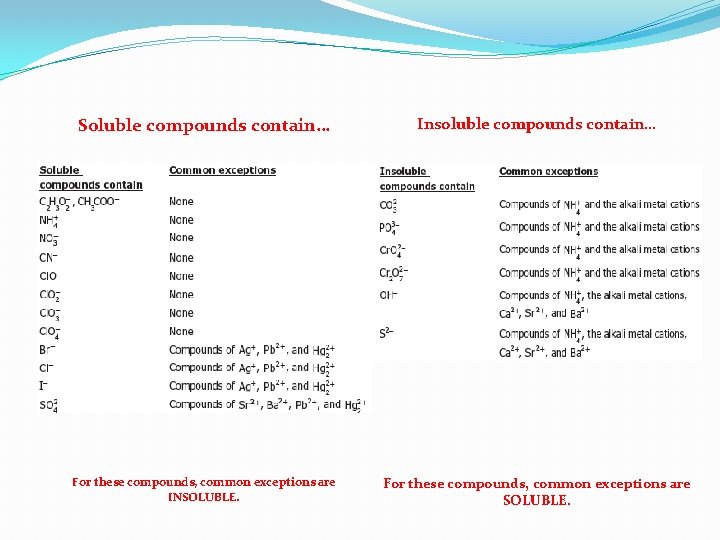
SOLUBLE OR INSOLUBLE? �Soluble: �able to be dissolved �Insoluble: �does not dissolve in solution (or water) �Precipitate: �an insoluble solid formed when two solutions are mixed

Soluble compounds contain… Insoluble compounds contain… For these compounds, common exceptions are INSOLUBLE. For these compounds, common exceptions are SOLUBLE.

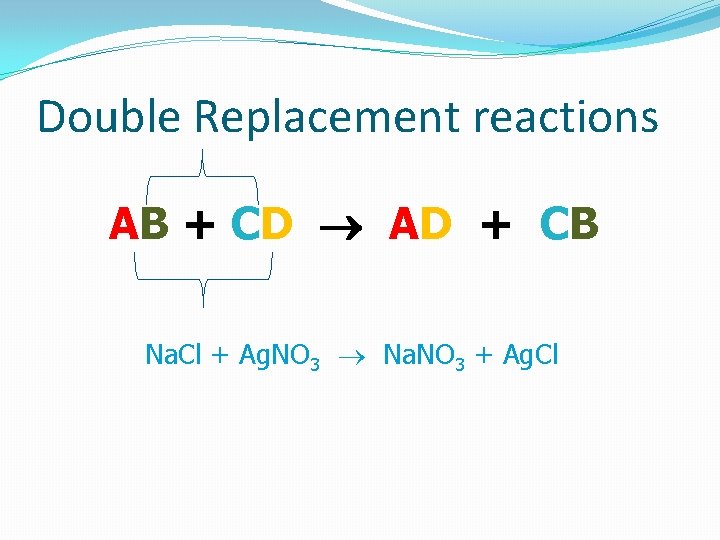
Double Replacement reactions AB + CD AD + CB Na. Cl + Ag. NO 3 Na. NO 3 + Ag. Cl

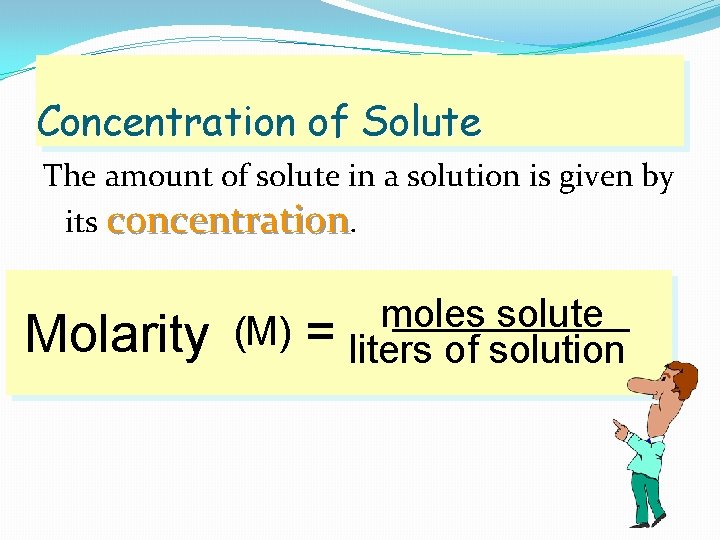
Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution

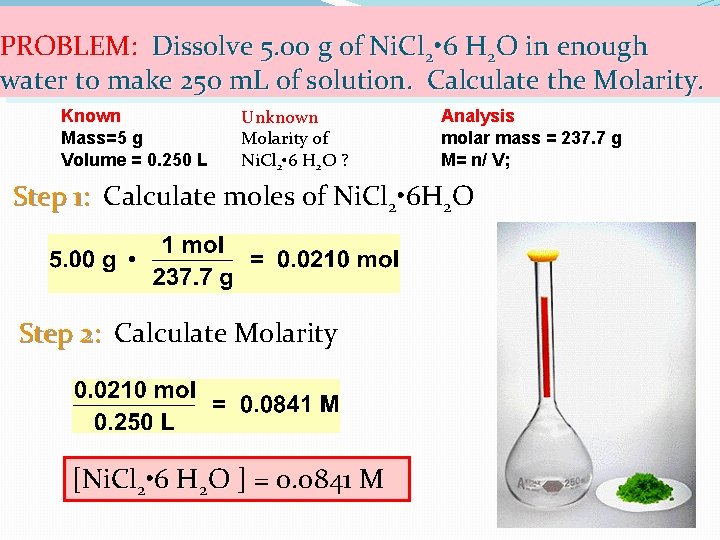
PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate the Molarity. Known Mass=5 g Volume = 0. 250 L Unknown Molarity of Ni. Cl 2 • 6 H 2 O ? Analysis molar mass = 237. 7 g M= n/ V; Step 1: Calculate moles of Ni. Cl 2 • 6 H 2 O Step 2: Calculate Molarity [Ni. Cl 2 • 6 H 2 O ] = 0. 0841 M

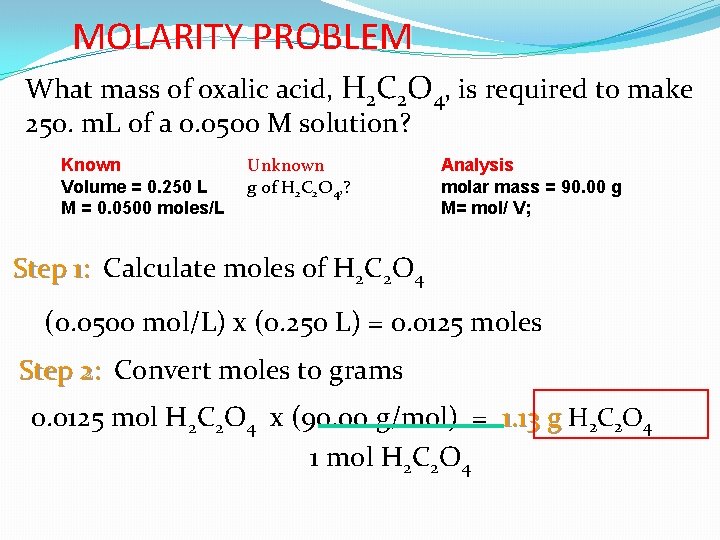
MOLARITY PROBLEM What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? Known Volume = 0. 250 L M = 0. 0500 moles/L Unknown g of H 2 C 2 O 4, ? Analysis molar mass = 90. 00 g M= mol/ V; Step 1: Calculate moles of H 2 C 2 O 4 (0. 0500 mol/L) x (0. 250 L) = 0. 0125 moles Step 2: Convert moles to grams 0. 0125 mol H 2 C 2 O 4 x (90. 00 g/mol) = 1. 13 g H 2 C 2 O 4 1 mol H 2 C 2 O 4

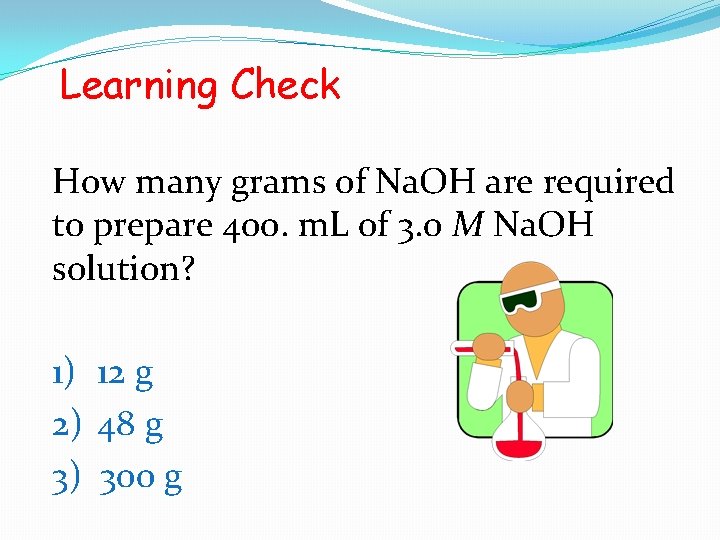
Learning Check How many grams of Na. OH are required to prepare 400. m. L of 3. 0 M Na. OH solution? 1) 12 g 2) 48 g 3) 300 g