Solutions and Water Structure Properties of solutions Water



- Slides: 22

Solutions and Water Structure

• Properties of solutions • Water microstructure • Solute microstructure – Ionic solutes – Polar solutes – Nonpolar solutes (the hydrophobic effect)


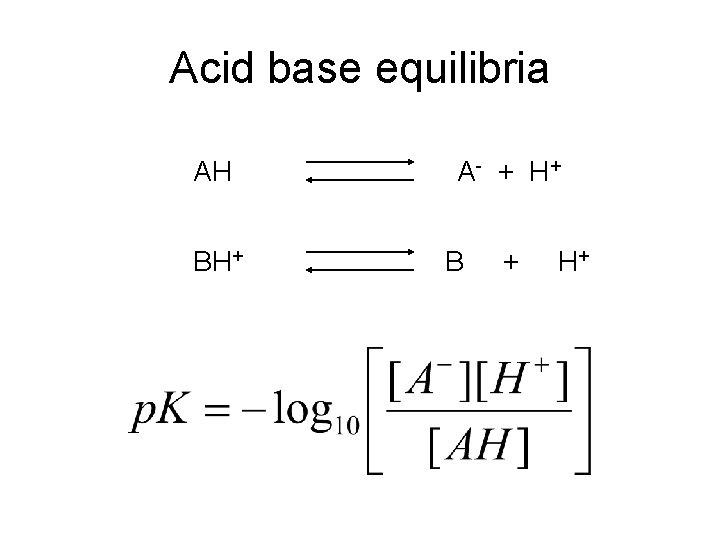
Acid base equilibria AH BH+ A- + H + B + H+

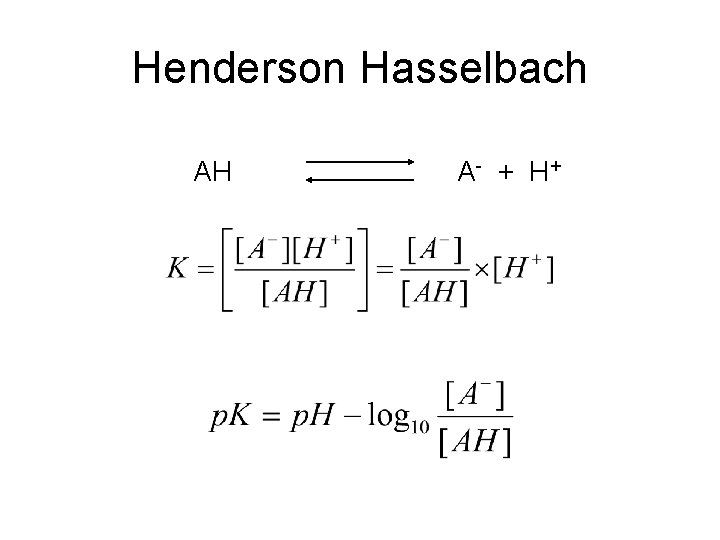
Henderson Hasselbach AH A- + H +

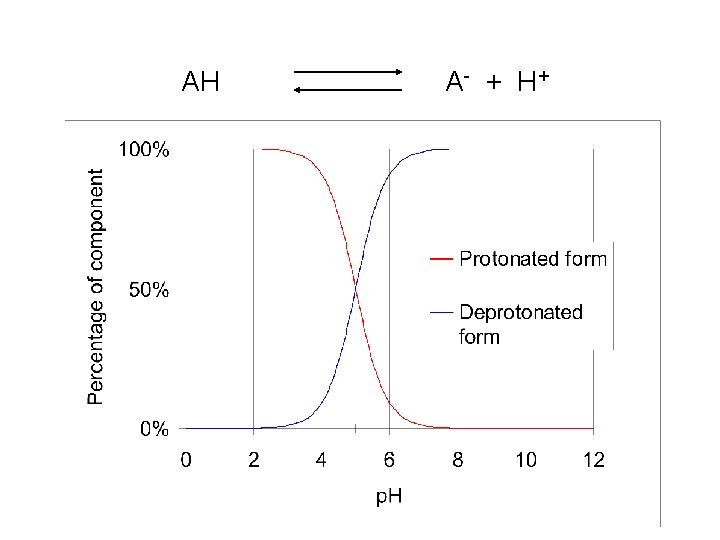
AH A- + H +

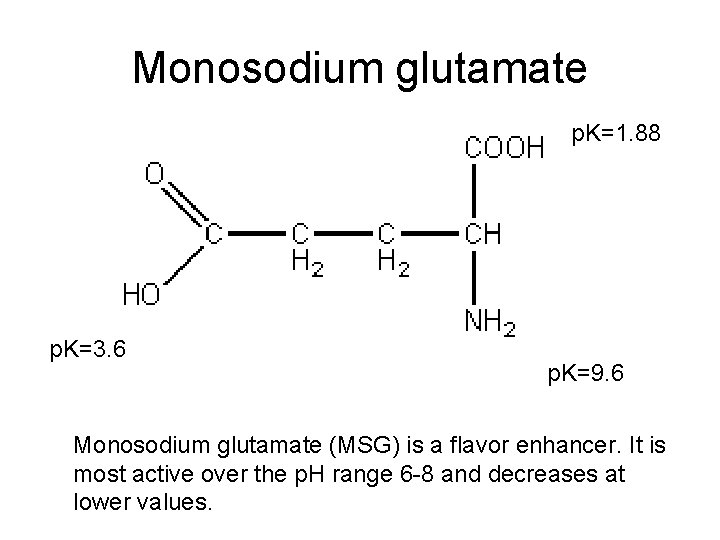
Monosodium glutamate p. K=1. 88 p. K=3. 6 p. K=9. 6 Monosodium glutamate (MSG) is a flavor enhancer. It is most active over the p. H range 6 -8 and decreases at lower values.

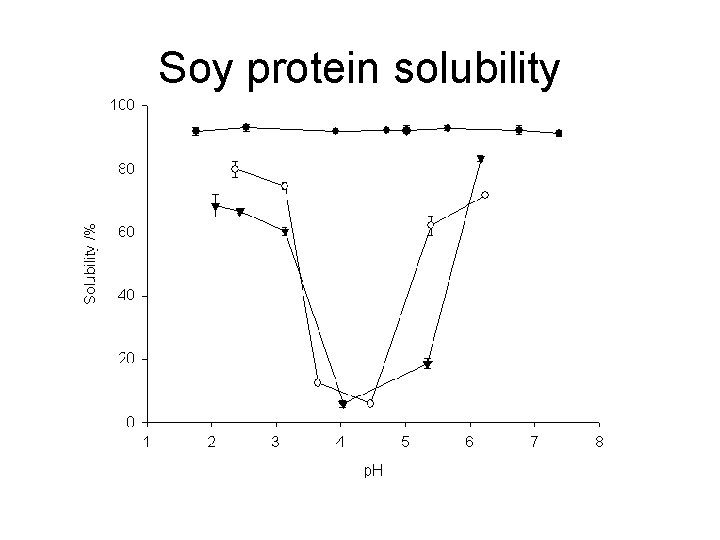
Soy protein solubility

Water Structure • Molecular structure • Supramolecular structure • Solutes – Ionic – Polar – Nonpolar (the hydrophobic effect)

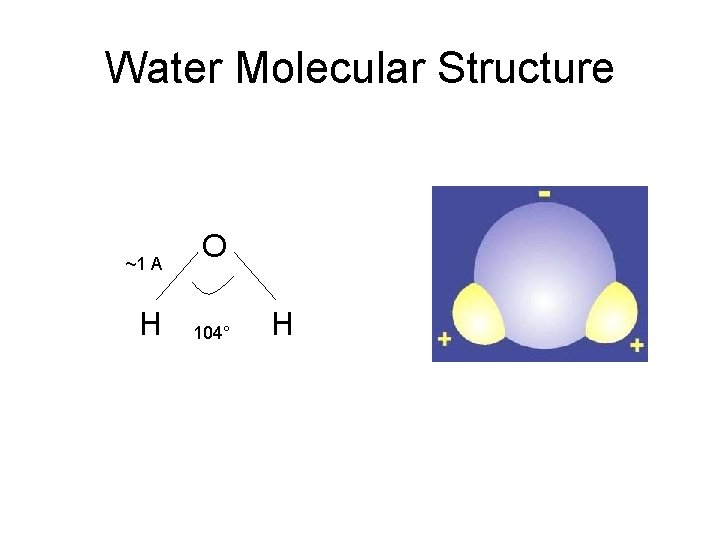
Water Molecular Structure ~1 A H O 104° H

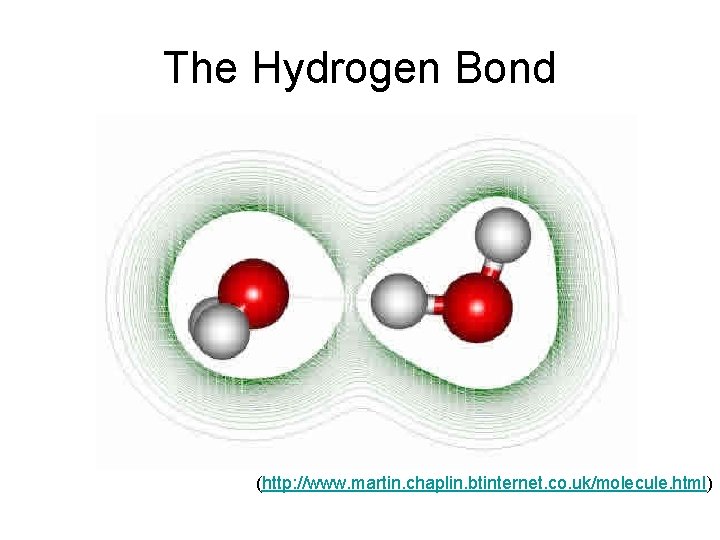
The Hydrogen Bond (http: //www. martin. chaplin. btinternet. co. uk/molecule. html)

Tetrahedral Structure of Water • The lone pairs and bonding electrons repel one another • The OH bonds are highly polarized • Strong H-bonds (~10% of covalent bond) • Each water molecule can hydrogen bond to two neighbors allowing the formation of an extensive 3 D structure • http: //wps. prenhall. com/wps/media/objects/439/4 49969/Media_Portfolio/Chapter_08/FG 08_13. JP G

Supramolecular Structure of Water • Water is highly hydrogen bonded (only about 15% of H-bonds break on melting ice) • The bonds form and break dynamically • Strong affinity of water for itself • High specific heat (to warm water must break some H-bonds)

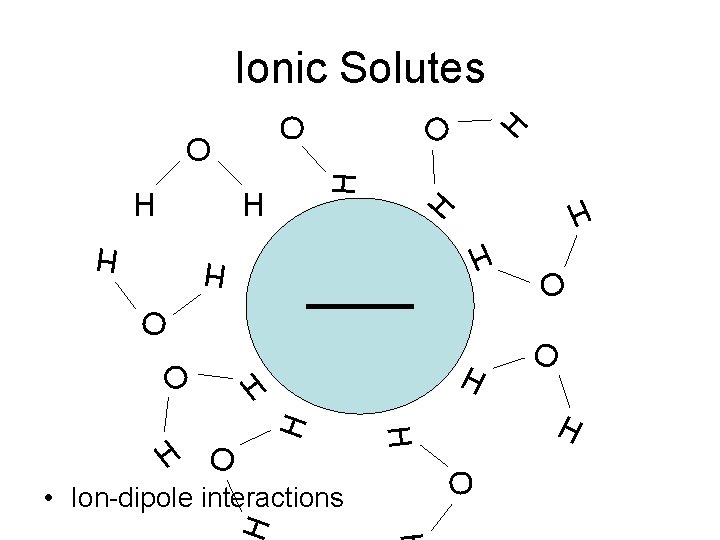
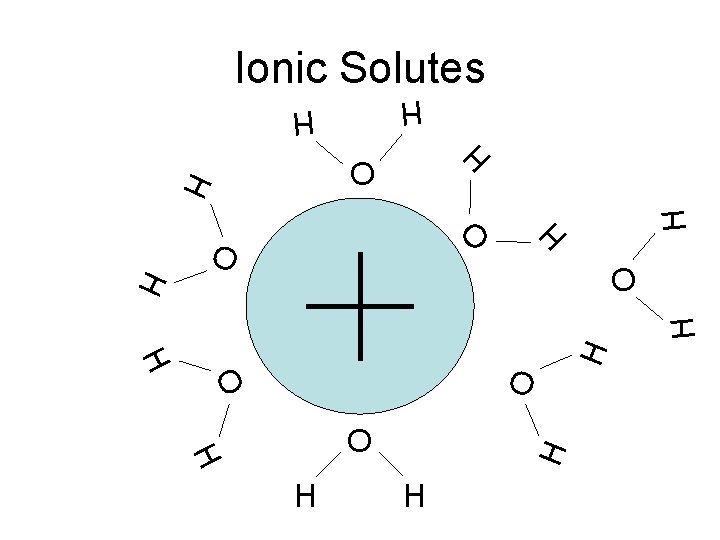
H H H O O • Ion-dipole interactions O H O O H H H O O O H Ionic Solutes H

Ionic Solutes H O H H O O H H H H O H

Polar Solutes • Hydrogen bonds • Dipole-dipole attractions

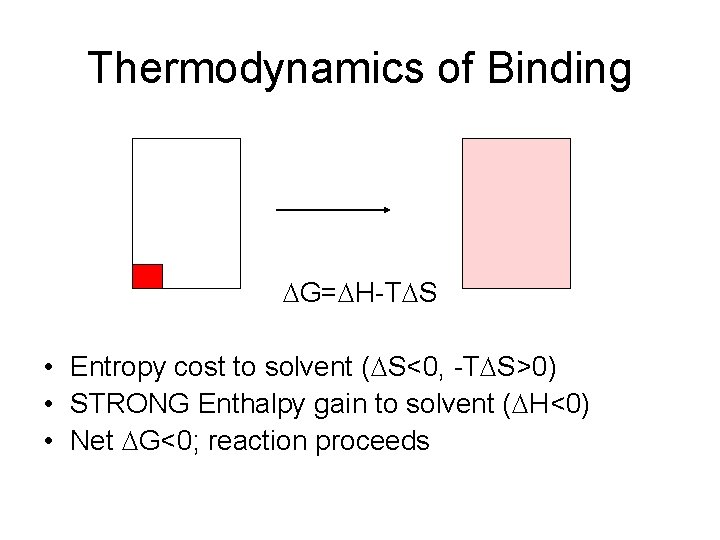
Thermodynamics of Binding DG=DH-TDS • Entropy cost to solvent (DS<0, -TDS>0) • STRONG Enthalpy gain to solvent (DH<0) • Net DG<0; reaction proceeds

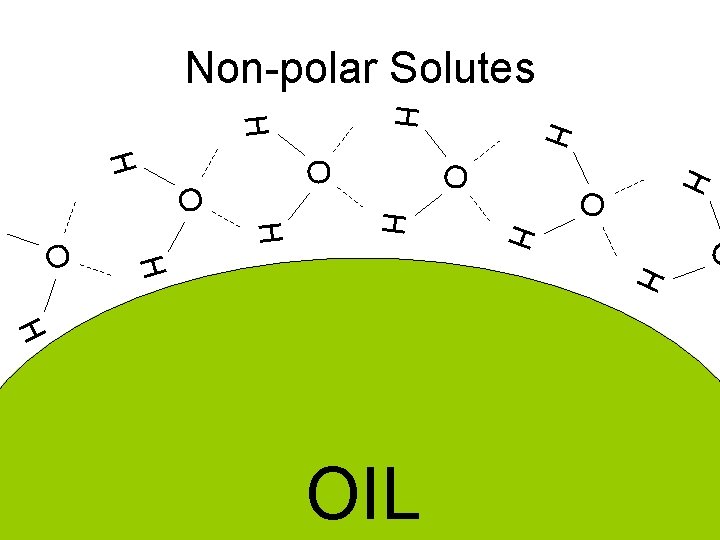
Non-polar Solutes H H O O H H H O H OIL

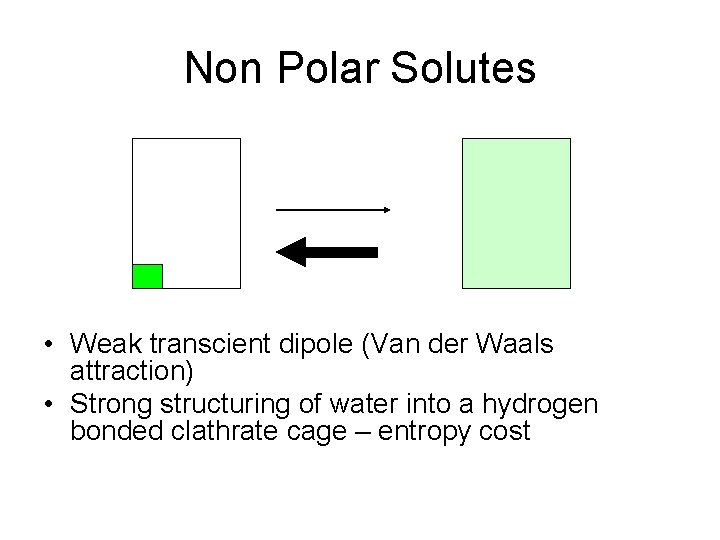
Non Polar Solutes • Weak transcient dipole (Van der Waals attraction) • Strong structuring of water into a hydrogen bonded clathrate cage – entropy cost

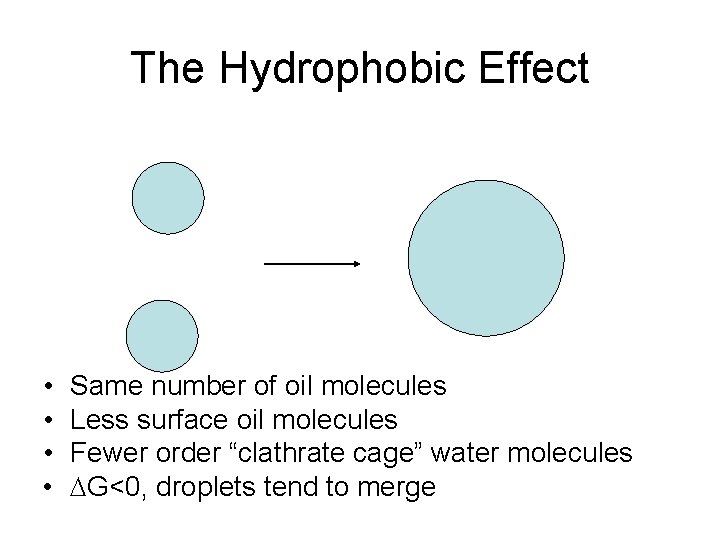
The Hydrophobic Effect • • Same number of oil molecules Less surface oil molecules Fewer order “clathrate cage” water molecules DG<0, droplets tend to merge

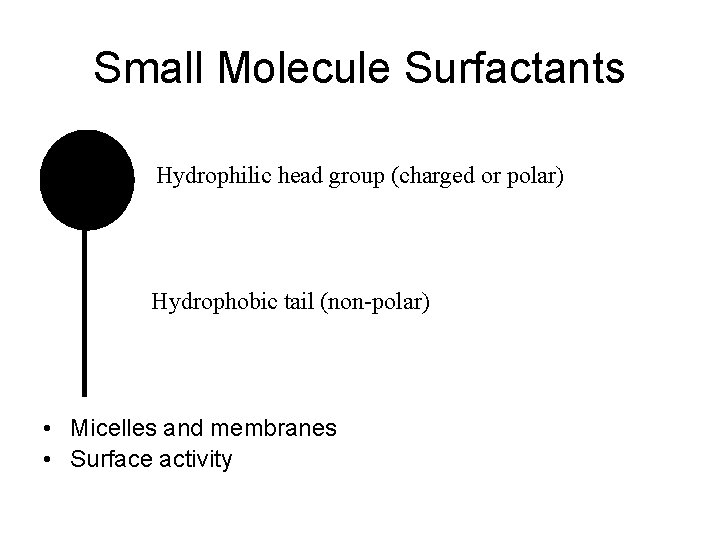
Small Molecule Surfactants Hydrophilic head group (charged or polar) Hydrophobic tail (non-polar) • Micelles and membranes • Surface activity

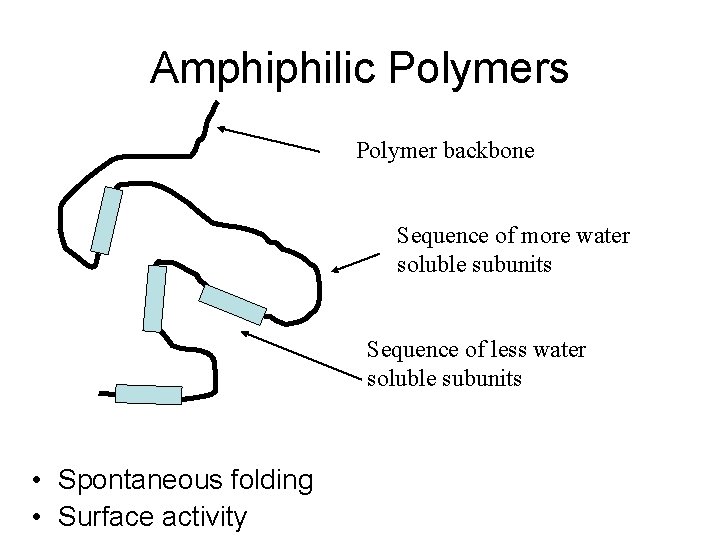
Amphiphilic Polymers Polymer backbone Sequence of more water soluble subunits Sequence of less water soluble subunits • Spontaneous folding • Surface activity
 Water and water and water water
Water and water and water water Intensive property and extensive properties
Intensive property and extensive properties Physical and chemical properties
Physical and chemical properties Freezing point chapter 13
Freezing point chapter 13 Water chemical and physical properties
Water chemical and physical properties Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Study of composition structure and properties
Study of composition structure and properties Wjec periodic table
Wjec periodic table Structure and properties of ceramics
Structure and properties of ceramics Polyethylene structure and properties
Polyethylene structure and properties Reverse mortgage solutions reo properties
Reverse mortgage solutions reo properties Physical properties of solutions
Physical properties of solutions Saturated and unsaturated solutions grade 7
Saturated and unsaturated solutions grade 7 Three colligative properties
Three colligative properties General properties of aqueous solutions
General properties of aqueous solutions Visitor management solutions for properties
Visitor management solutions for properties Physical properties of solutions
Physical properties of solutions Chapter 13 properties of solutions
Chapter 13 properties of solutions Chemistry in biology section 3 water and solutions
Chemistry in biology section 3 water and solutions Water properties concept map
Water properties concept map Properties of water lab ap biology
Properties of water lab ap biology Physical properties of sea water
Physical properties of sea water Properties of water clipart
Properties of water clipart