Redox Reactions and Electrochemistry Chapter 19 Voltaic Cells



![Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0.](https://slidetodoc.com/presentation_image_h2/c7f85bd509d7757e946c01f2edec6469/image-25.jpg)
- Slides: 25

Redox Reactions and Electrochemistry Chapter 19

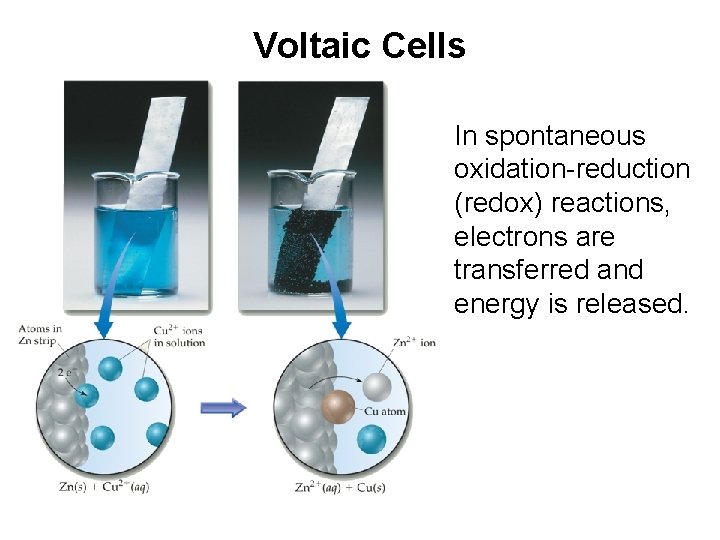
Voltaic Cells In spontaneous oxidation-reduction (redox) reactions, electrons are transferred and energy is released.

Voltaic Cells • We can use that energy to do work if we make the electrons flow through an external device. • We call such a setup a voltaic cell, or a galvanic cell. • The particular arrangement of electrodes (Zn and Cu) and solutions (Zn. SO 4 and Cu. SO 4) is called a Daniell cell.

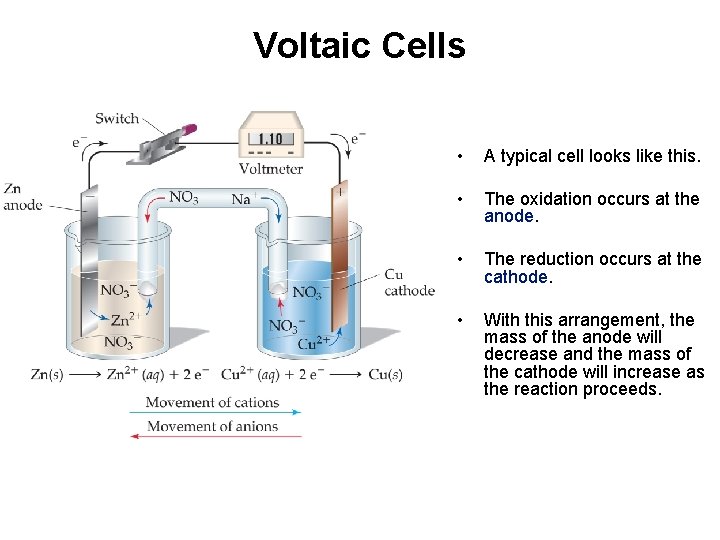
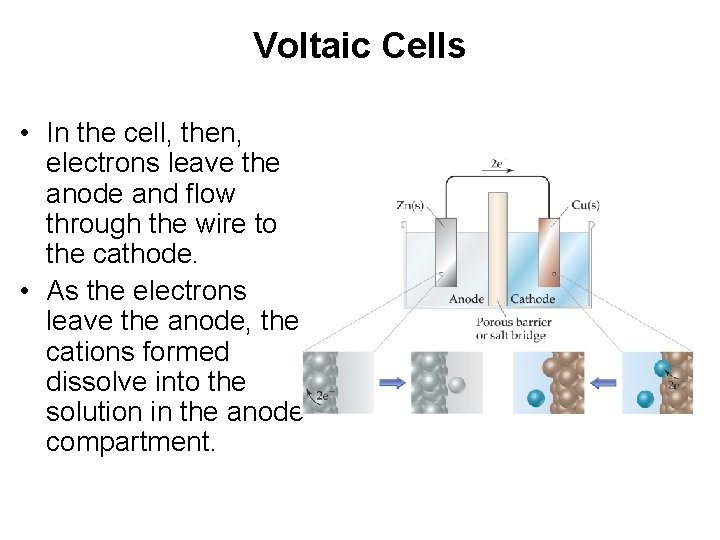
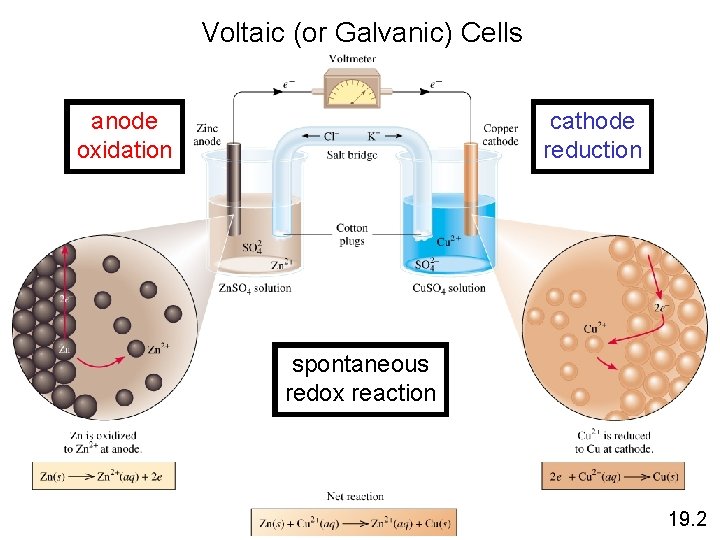
Voltaic Cells • A typical cell looks like this. • The oxidation occurs at the anode. • The reduction occurs at the cathode. • With this arrangement, the mass of the anode will decrease and the mass of the cathode will increase as the reaction proceeds.

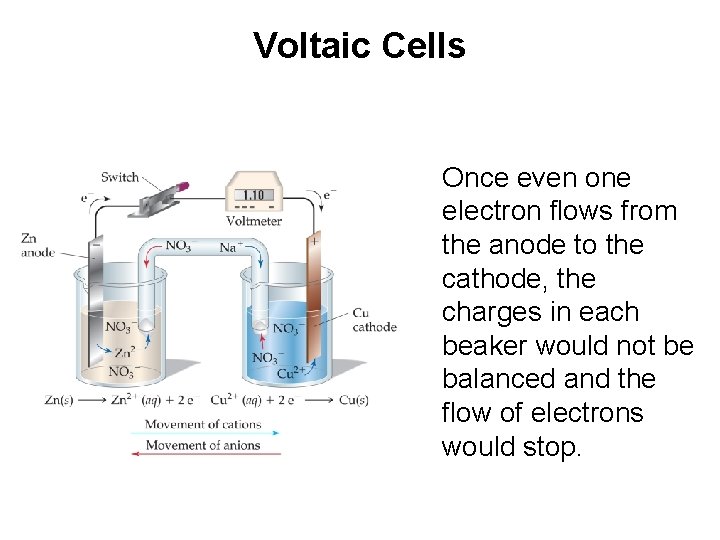
Voltaic Cells Once even one electron flows from the anode to the cathode, the charges in each beaker would not be balanced and the flow of electrons would stop.

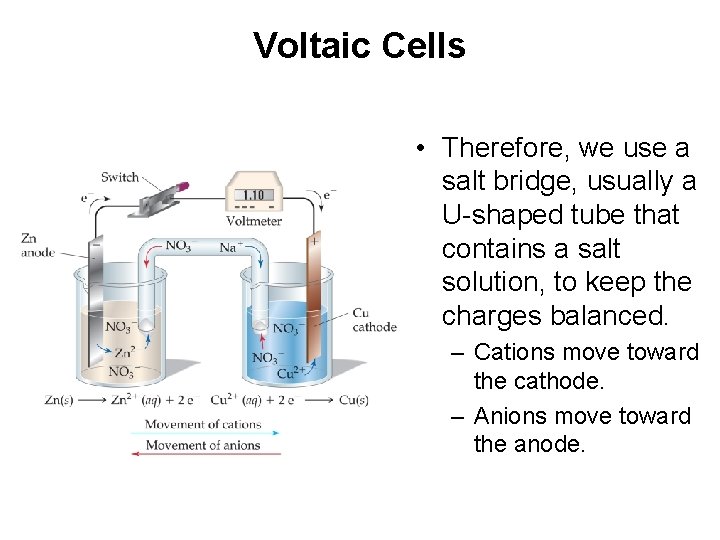
Voltaic Cells • Therefore, we use a salt bridge, usually a U-shaped tube that contains a salt solution, to keep the charges balanced. – Cations move toward the cathode. – Anions move toward the anode.

Voltaic Cells • In the cell, then, electrons leave the anode and flow through the wire to the cathode. • As the electrons leave the anode, the cations formed dissolve into the solution in the anode compartment.

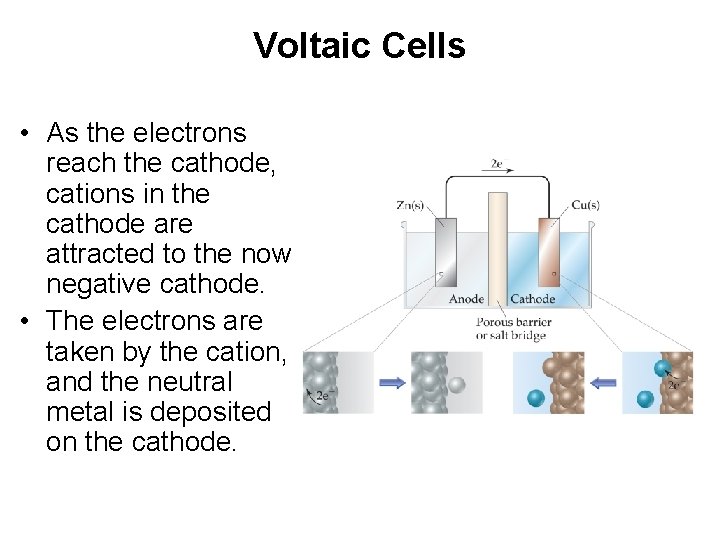
Voltaic Cells • As the electrons reach the cathode, cations in the cathode are attracted to the now negative cathode. • The electrons are taken by the cation, and the neutral metal is deposited on the cathode.

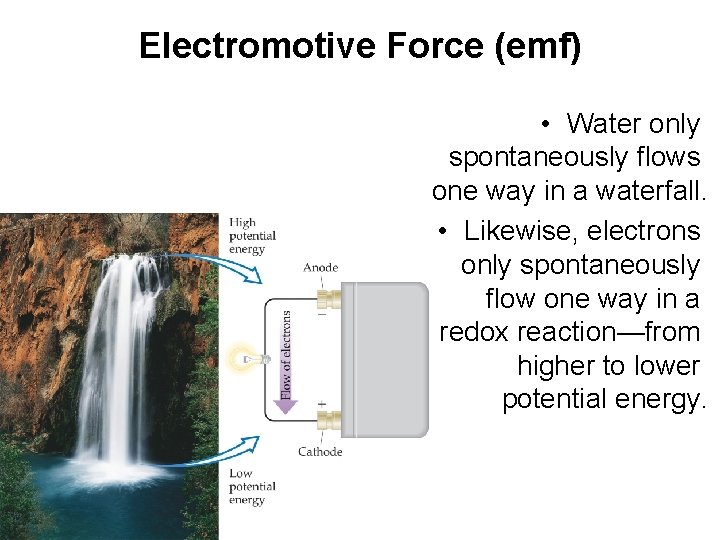
Electromotive Force (emf) • Water only spontaneously flows one way in a waterfall. • Likewise, electrons only spontaneously flow one way in a redox reaction—from higher to lower potential energy.

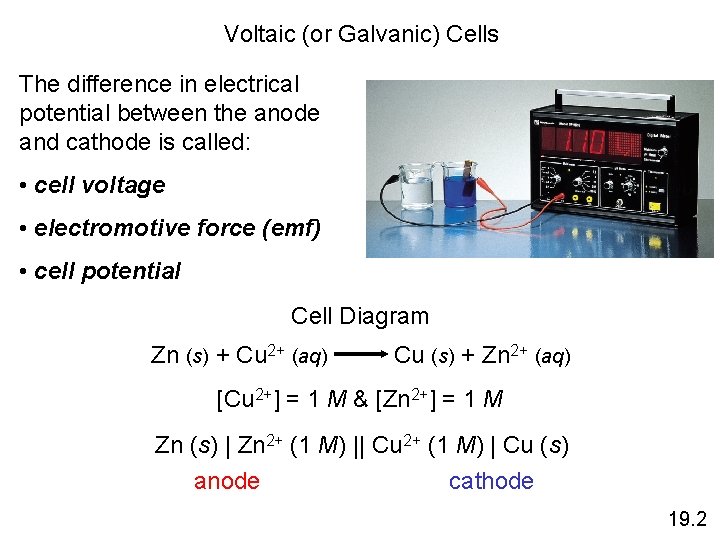
Electromotive Force (emf) • The potential difference between the anode and cathode in a cell is called the electromotive force (emf). • It is also called the cell potential, and is designated Ecell.

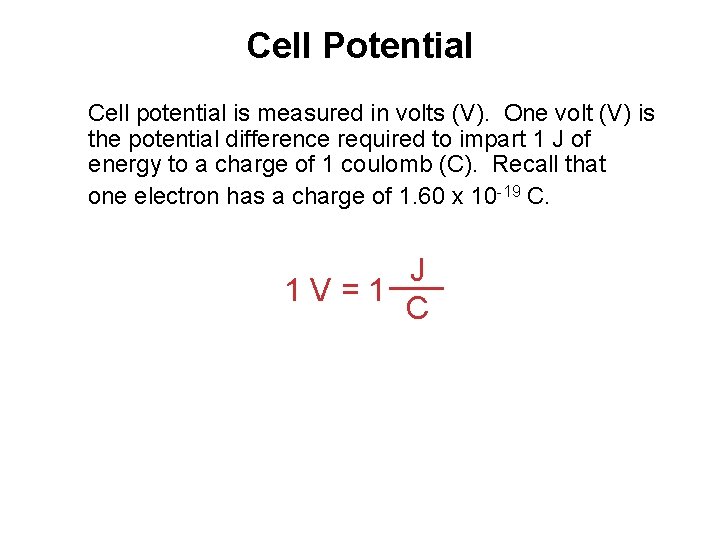
Cell Potential Cell potential is measured in volts (V). One volt (V) is the potential difference required to impart 1 J of energy to a charge of 1 coulomb (C). Recall that one electron has a charge of 1. 60 x 10 -19 C. J 1 V=1 C

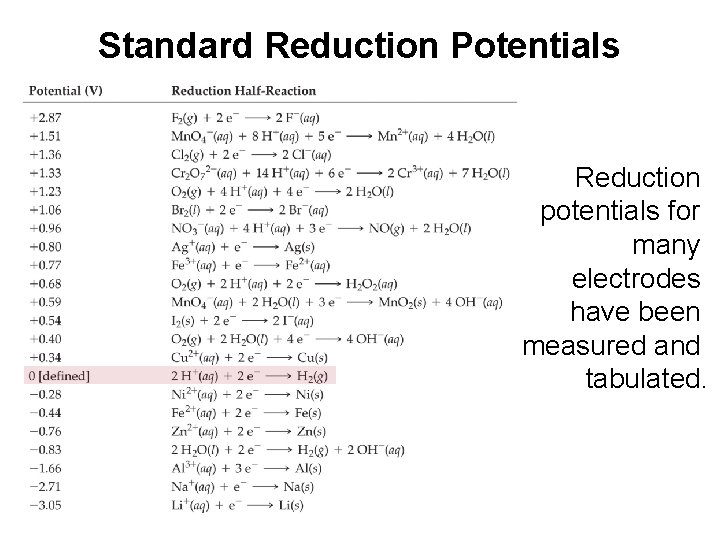
Standard Reduction Potentials Reduction potentials for many electrodes have been measured and tabulated.

Voltaic (or Galvanic) Cells anode oxidation cathode reduction spontaneous redox reaction 19. 2

Voltaic (or Galvanic) Cells The difference in electrical potential between the anode and cathode is called: • cell voltage • electromotive force (emf) • cell potential Cell Diagram Zn (s) + Cu 2+ (aq) Cu (s) + Zn 2+ (aq) [Cu 2+] = 1 M & [Zn 2+] = 1 M Zn (s) | Zn 2+ (1 M) || Cu 2+ (1 M) | Cu (s) anode cathode 19. 2

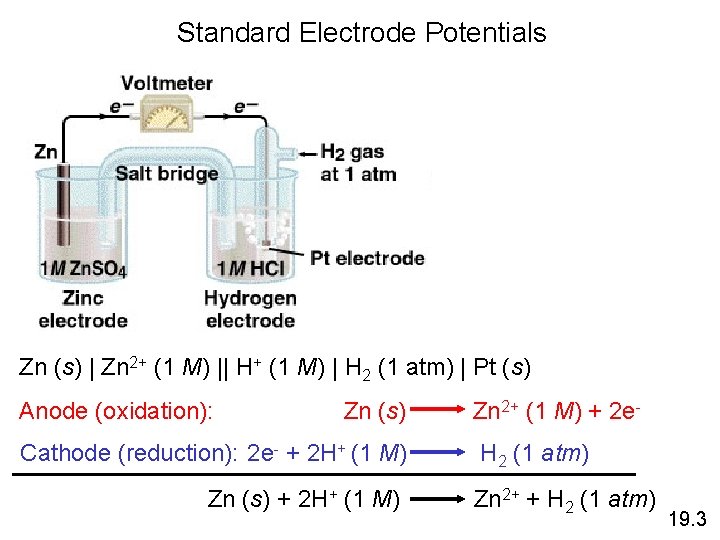
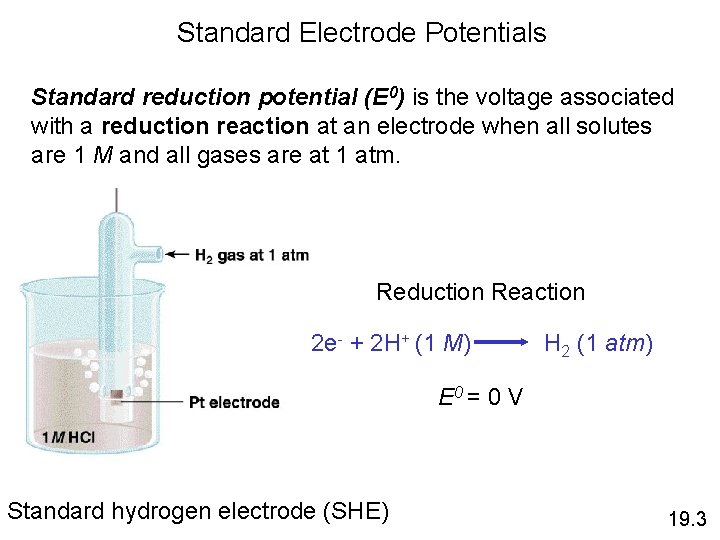
Standard Electrode Potentials Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) Anode (oxidation): Zn (s) Cathode (reduction): 2 e- + 2 H+ (1 M) Zn (s) + 2 H+ (1 M) Zn 2+ (1 M) + 2 e. H 2 (1 atm) Zn 2+ + H 2 (1 atm) 19. 3

Standard Electrode Potentials Standard reduction potential (E 0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Reduction Reaction 2 e- + 2 H+ (1 M) H 2 (1 atm) E 0 = 0 V Standard hydrogen electrode (SHE) 19. 3

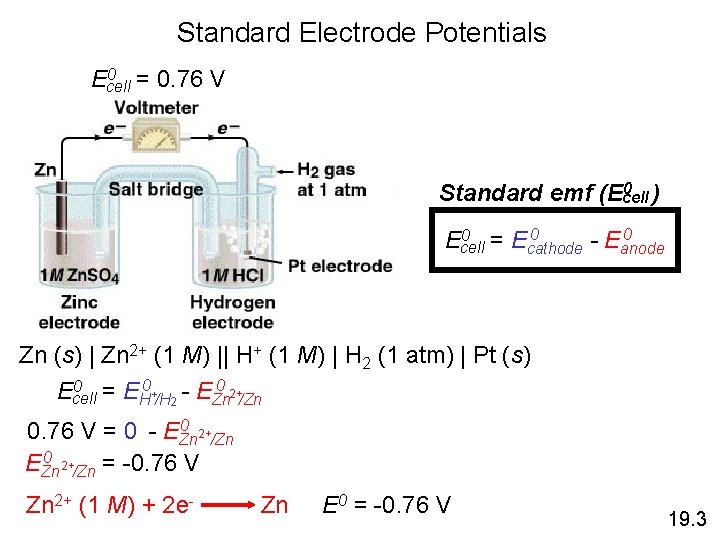
Standard Electrode Potentials 0 = 0. 76 V Ecell 0 ) Standard emf (Ecell 0 0 = E 0 Ecell cathode - Eanode Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) 0 = E 0 + - E 0 2+ Ecell H /H 2 Zn /Zn 0 2+ 0. 76 V = 0 - EZn /Zn 0 2+ EZn /Zn = -0. 76 V Zn 2+ (1 M) + 2 e- Zn E 0 = -0. 76 V 19. 3

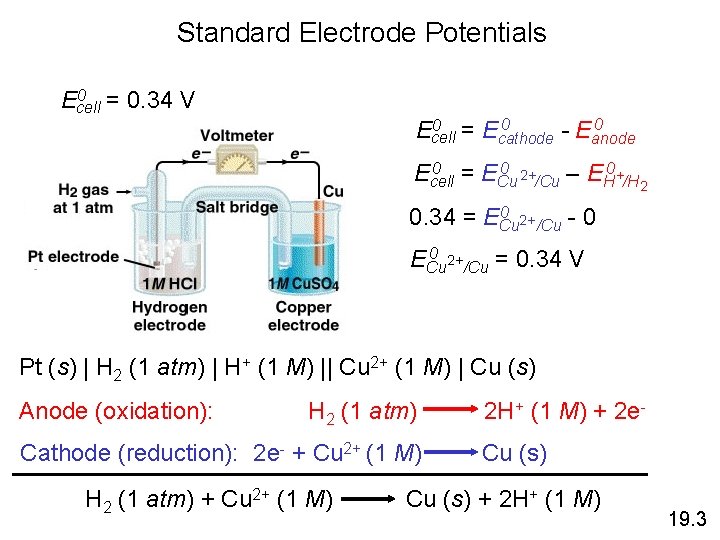
Standard Electrode Potentials 0 = 0. 34 V Ecell 0 0 = E 0 Ecell cathode - Eanode 0 = E 0 2+ 0 Ecell Cu /Cu – EH +/H 2 0 2+ 0. 34 = ECu /Cu - 0 0 2+ ECu /Cu = 0. 34 V Pt (s) | H 2 (1 atm) | H+ (1 M) || Cu 2+ (1 M) | Cu (s) Anode (oxidation): H 2 (1 atm) Cathode (reduction): 2 e- + Cu 2+ (1 M) H 2 (1 atm) + Cu 2+ (1 M) 2 H+ (1 M) + 2 e. Cu (s) + 2 H+ (1 M) 19. 3

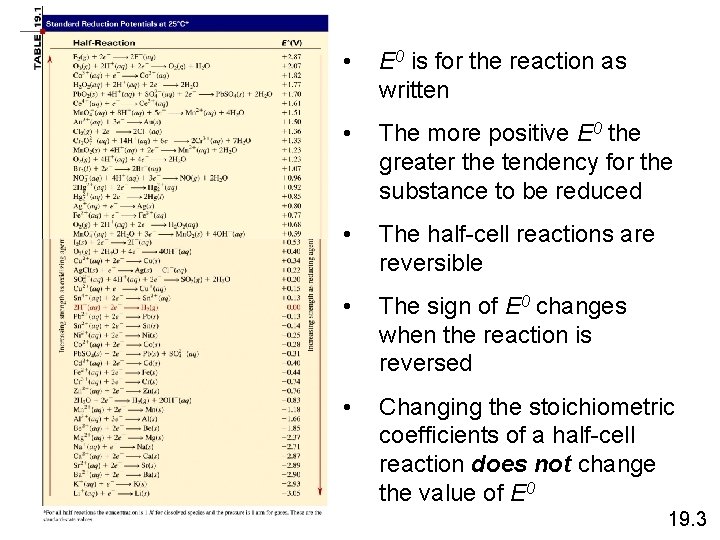
• E 0 is for the reaction as written • The more positive E 0 the greater the tendency for the substance to be reduced • The half-cell reactions are reversible • The sign of E 0 changes when the reaction is reversed • Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E 0 19. 3

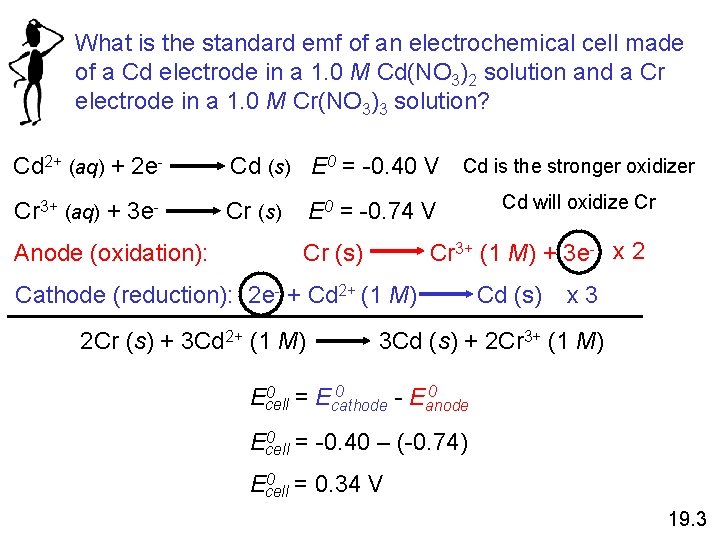
What is the standard emf of an electrochemical cell made of a Cd electrode in a 1. 0 M Cd(NO 3)2 solution and a Cr electrode in a 1. 0 M Cr(NO 3)3 solution? Cd 2+ (aq) + 2 e- Cd (s) E 0 = -0. 40 V Cd is the stronger oxidizer Cr 3+ (aq) + 3 e- Cr (s) Anode (oxidation): E 0 = -0. 74 V Cr 3+ (1 M) + 3 e- x 2 Cr (s) Cathode (reduction): 2 e- + Cd 2+ (1 M) 2 Cr (s) + 3 Cd 2+ (1 M) Cd will oxidize Cr Cd (s) x 3 3 Cd (s) + 2 Cr 3+ (1 M) 0 0 = E 0 Ecell cathode - Eanode 0 = -0. 40 – (-0. 74) Ecell 0 = 0. 34 V Ecell 19. 3

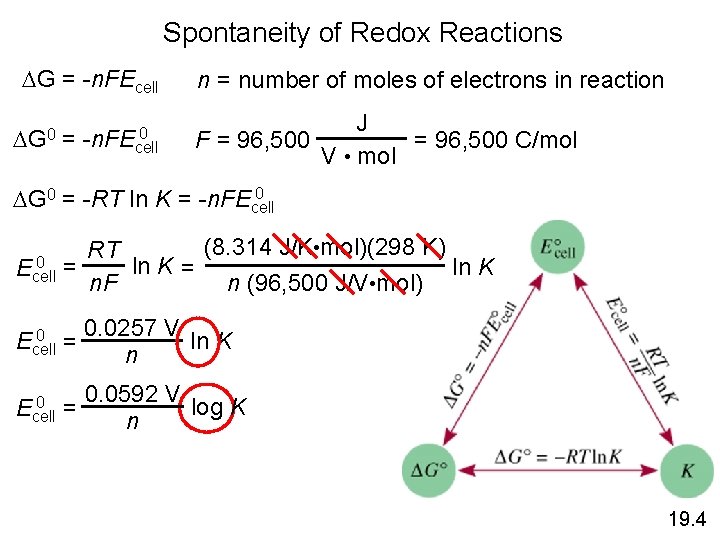
Spontaneity of Redox Reactions DG = -n. FEcell DG 0 = 0 -n. FEcell n = number of moles of electrons in reaction J F = 96, 500 C/mol V • mol 0 DG 0 = -RT ln K = -n. FEcell (8. 314 J/K • mol)(298 K) RT 0 = ln K Ecell n. F n (96, 500 J/V • mol) 0 Ecell = 0 Ecell 0. 0257 V ln K n 0. 0592 V log K = n 19. 4

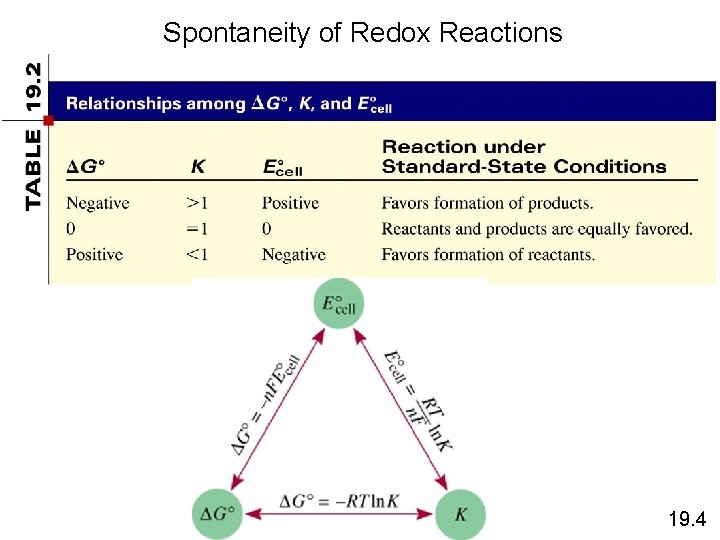
Spontaneity of Redox Reactions 19. 4

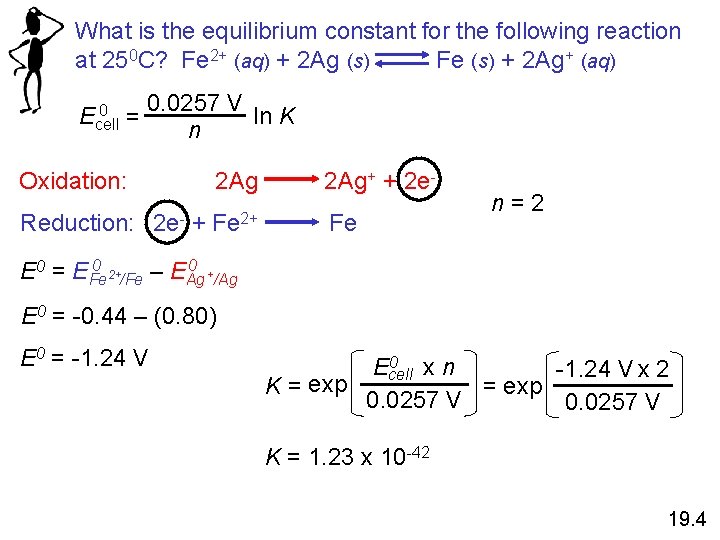
What is the equilibrium constant for the following reaction at 250 C? Fe 2+ (aq) + 2 Ag (s) Fe (s) + 2 Ag+ (aq) 0 Ecell = 0. 0257 V ln K n Oxidation: Reduction: 2 e- + 2 Ag+ + 2 e- Fe 2+ Fe n=2 0 0 E 0 = EFe 2+/Fe – EAg +/Ag E 0 = -0. 44 – (0. 80) E 0 = -1. 24 V 0 Ecell xn -1. 24 V x 2 exp = exp K= 0. 0257 V K = 1. 23 x 10 -42 19. 4

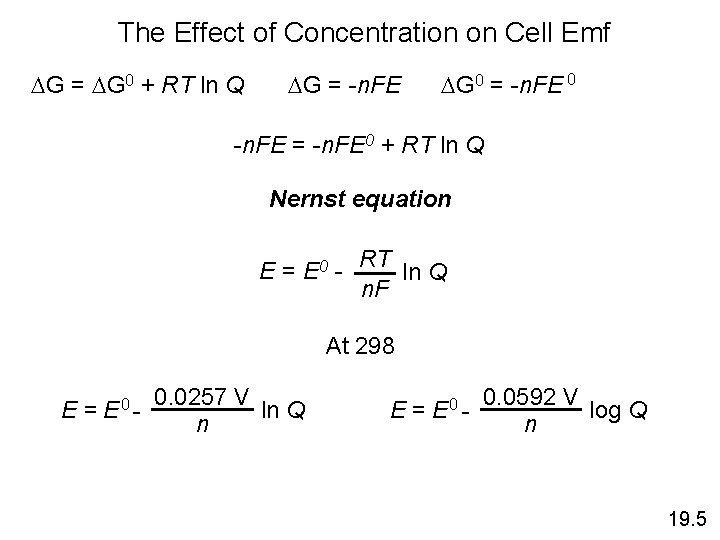
The Effect of Concentration on Cell Emf DG = DG 0 + RT ln Q DG = -n. FE DG 0 = -n. FE 0 -n. FE = -n. FE 0 + RT ln Q Nernst equation E = E 0 - RT ln Q n. F At 298 E = E 0 - 0. 0257 V ln Q n E = E 0 - 0. 0592 V log Q n 19. 5
![Will the following reaction occur spontaneously at 250 C if Fe 2 0 Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0.](https://slidetodoc.com/presentation_image_h2/c7f85bd509d7757e946c01f2edec6469/image-25.jpg)
Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. 60 M and [Cd 2+] = 0. 010 M? Fe 2+ (aq) + Cd (s) Fe (s) + Cd 2+ (aq) Oxidation: Reduction: Cd 2 e- + Cd 2+ + 2 e- Fe 2+ 2 Fe n=2 0 0 E 0 = EFe 2+/Fe – ECd 2+/Cd E 0 = -0. 44 – (-0. 40) E 0 = -0. 04 V 0. 0257 V ln Q n 0. 010 0. 0257 V ln E = -0. 04 V 2 0. 60 E = 0. 013 E = E 0 - E>0 Spontaneous 19. 5