Chapter A 2 2 4 Voltaic Cells Voltaic










- Slides: 41

Chapter A 2 2. 4 – Voltaic Cells

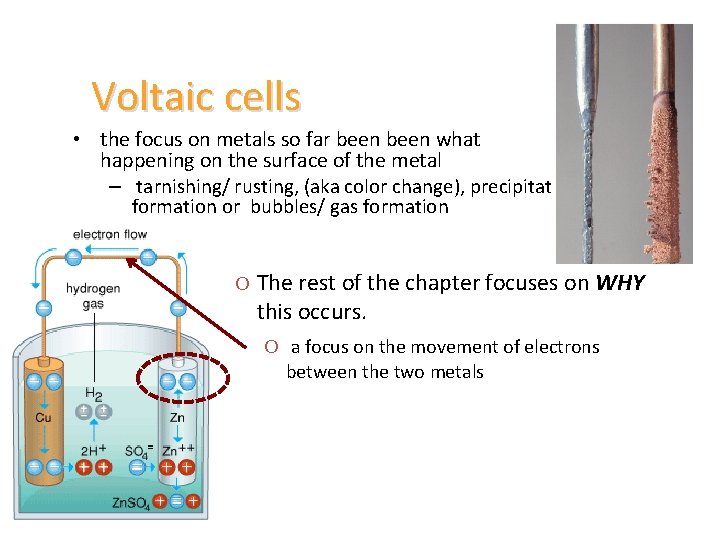
Voltaic cells • the focus on metals so far been what happening on the surface of the metal – tarnishing/ rusting, (aka color change), precipitate formation or bubbles/ gas formation O The rest of the chapter focuses on WHY this occurs. O a focus on the movement of electrons between the two metals

Voltaic cells • though commonly a voltaic cell is referred to as a “battery”, technically cells are only referred to as a battery when several are together – when an electronic device is operating, voltaic cells provide a continuous flow (current) of electrons, which is converted into current to power the device

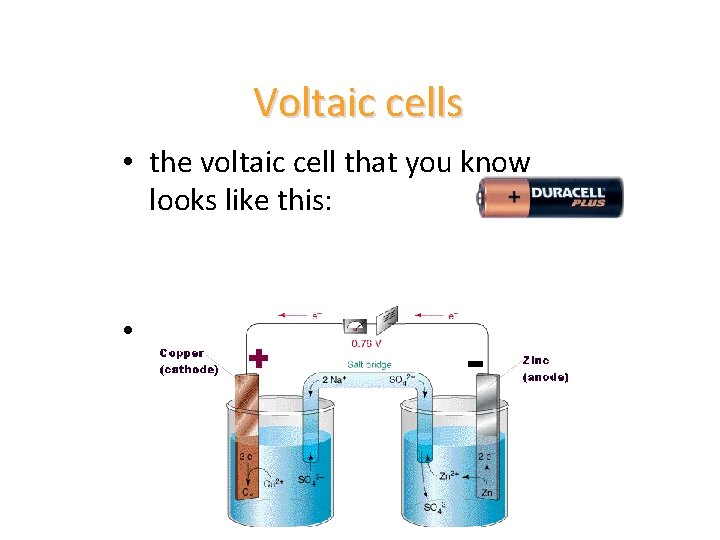
Voltaic cells • the voltaic cell that you know looks like this: • this is the version of the voltaic cell we will make in the lab.

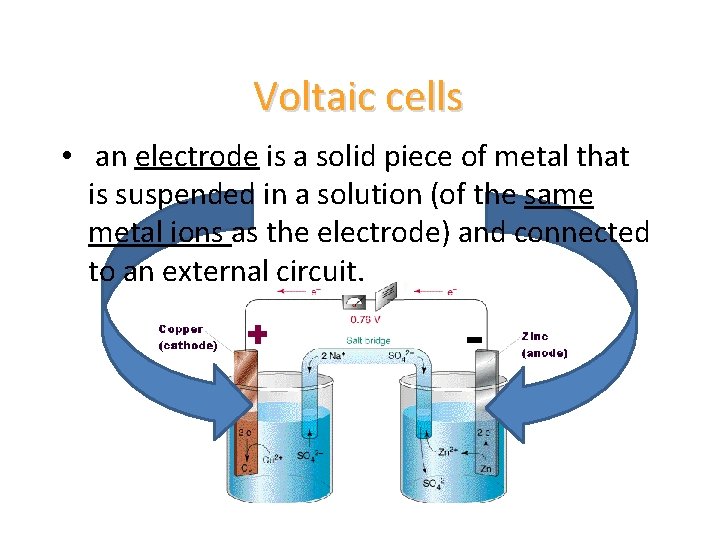
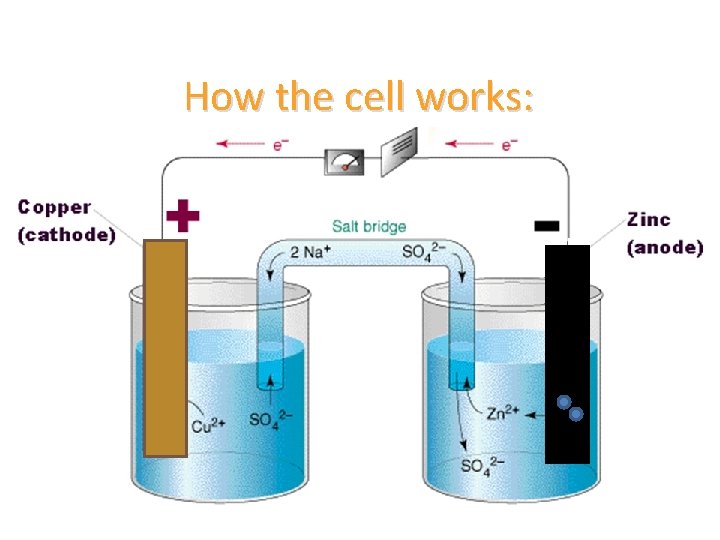
Voltaic cells • an electrode is a solid piece of metal that is suspended in a solution (of the same metal ions as the electrode) and connected to an external circuit.

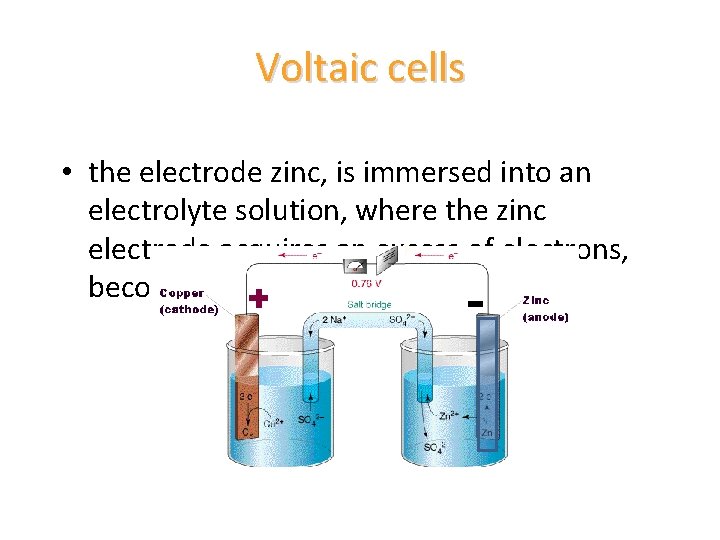
Voltaic cells • the electrode zinc, is immersed into an electrolyte solution, where the zinc electrode acquires an excess of electrons, becoming negatively charged

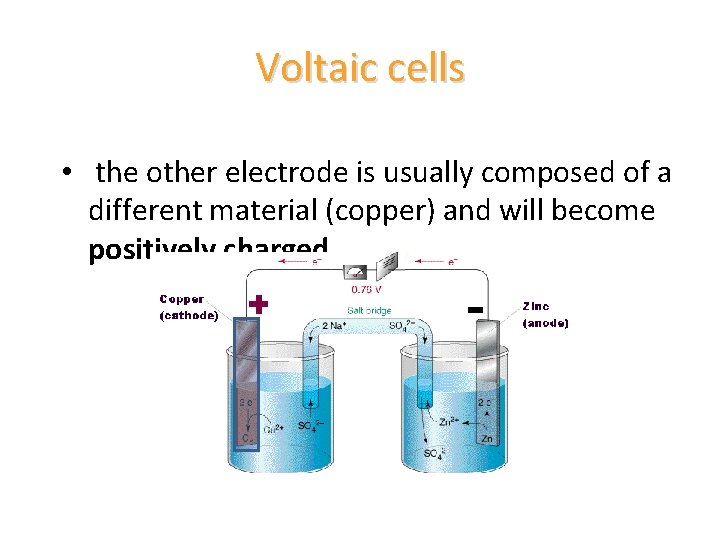
Voltaic cells • the other electrode is usually composed of a different material (copper) and will become positively charged

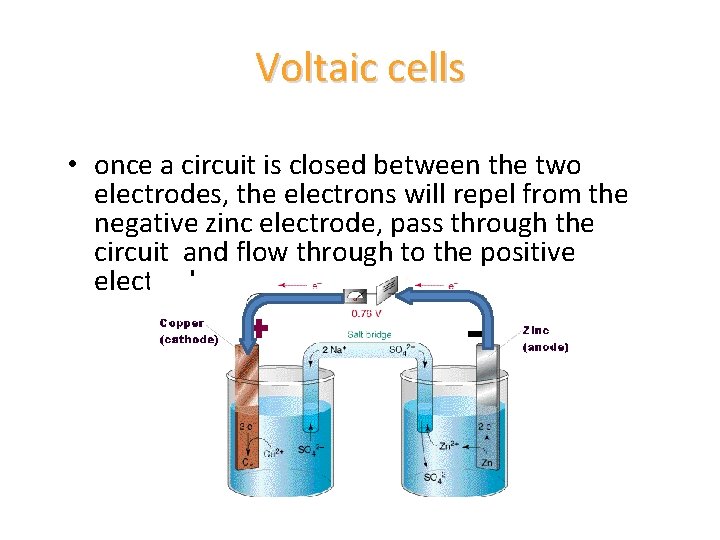
Voltaic cells • once a circuit is closed between the two electrodes, the electrons will repel from the negative zinc electrode, pass through the circuit and flow through to the positive electrode

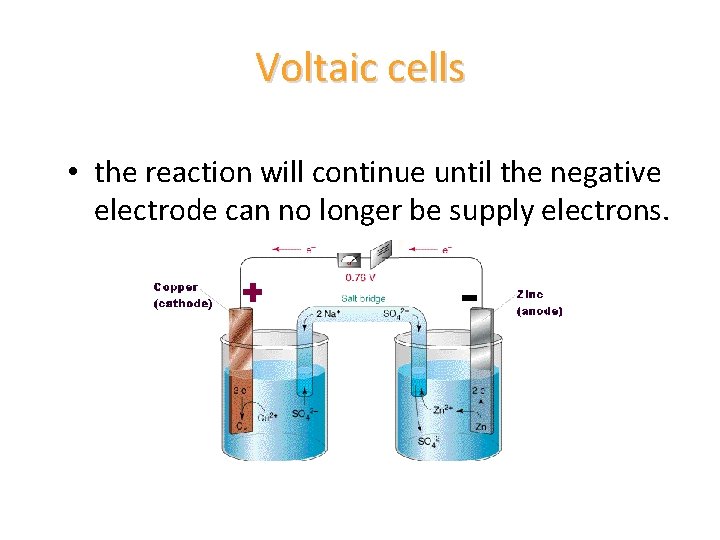
Voltaic cells • the reaction will continue until the negative electrode can no longer be supply electrons.

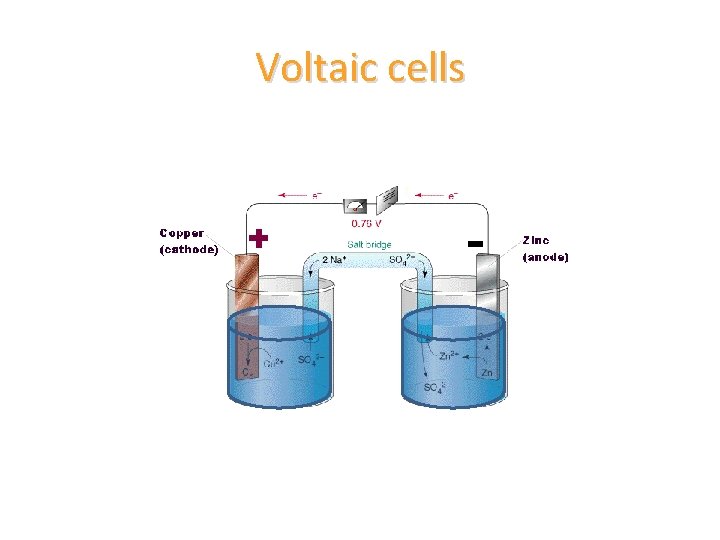
Voltaic cells

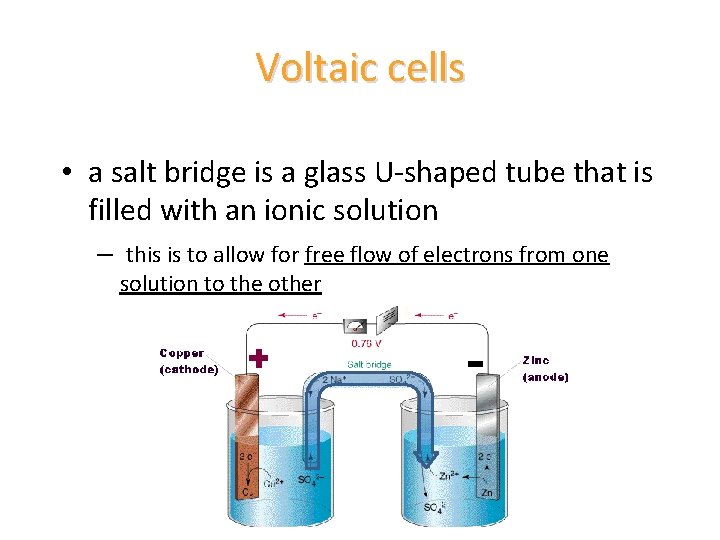
Voltaic cells • a salt bridge is a glass U-shaped tube that is filled with an ionic solution – this is to allow for free flow of electrons from one solution to the other

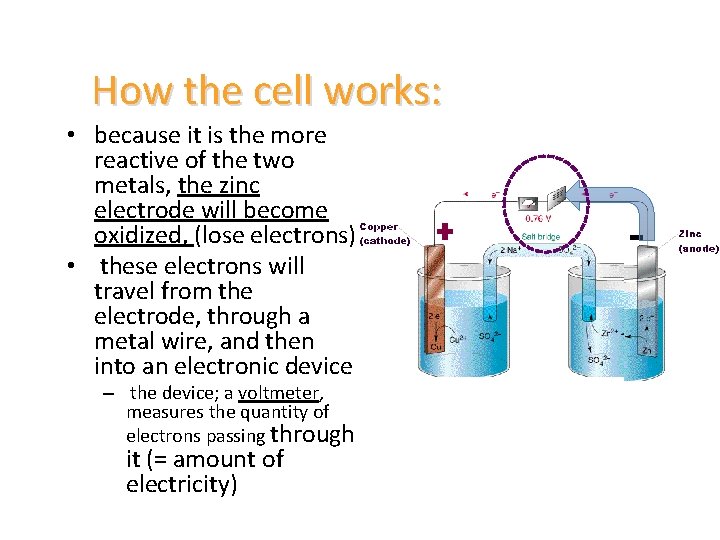
How the cell works: • because it is the more reactive of the two metals, the zinc electrode will become oxidized, (lose electrons) • these electrons will travel from the electrode, through a metal wire, and then into an electronic device – the device; a voltmeter, measures the quantity of electrons passing through it (= amount of electricity)

How the cell works: • the electrons will pass through the device, back through another wire, into the copper electrode – these electrons will be attracted to the Cu 2+(aq) ions in the solution and they will be reduced. • over time, the zinc electrode shrinks in size (as Zn 2+) and the copper electrode grows (Cu 2+ Cu)

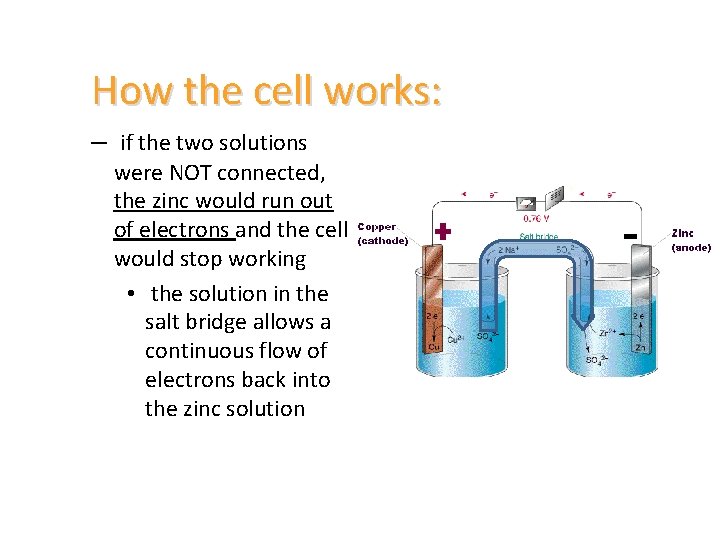
How the cell works: – if the two solutions were NOT connected, the zinc would run out of electrons and the cell would stop working • the solution in the salt bridge allows a continuous flow of electrons back into the zinc solution

How the cell works:

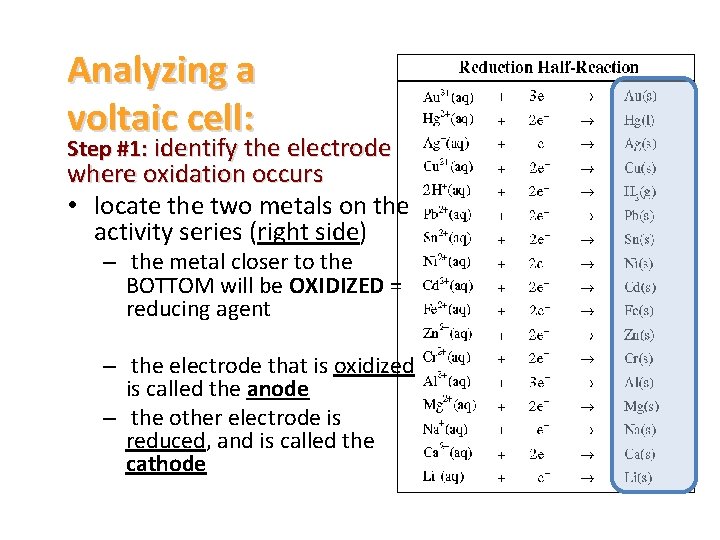
Analyzing a voltaic cell: Step #1: identify the electrode where oxidation occurs • locate the two metals on the activity series (right side) – the metal closer to the BOTTOM will be OXIDIZED = reducing agent – the electrode that is oxidized is called the anode – the other electrode is reduced, and is called the cathode

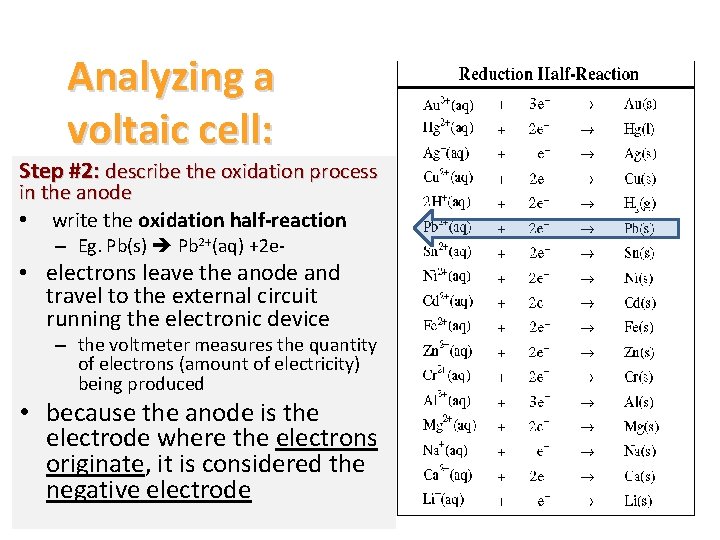
Analyzing a voltaic cell: Step #2: describe the oxidation process in the anode • write the oxidation half-reaction – Eg. Pb(s) Pb 2+(aq) +2 e- • electrons leave the anode and travel to the external circuit running the electronic device – the voltmeter measures the quantity of electrons (amount of electricity) being produced • because the anode is the electrode where the electrons originate, it is considered the negative electrode

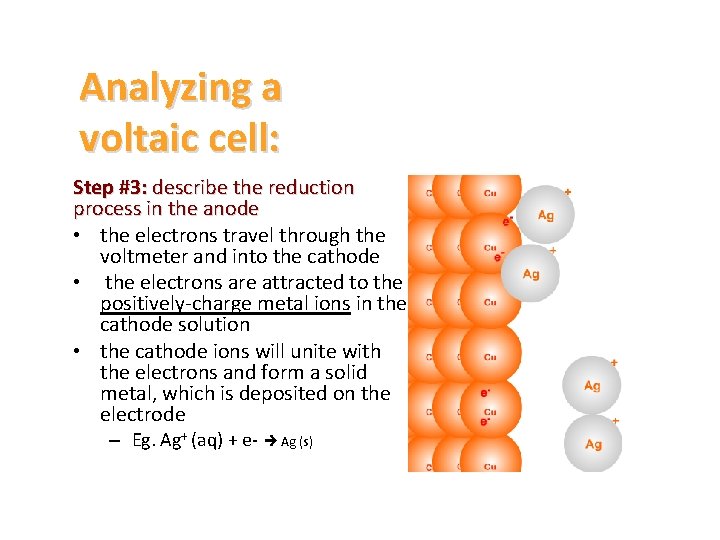
Analyzing a voltaic cell: Step #3: describe the reduction process in the anode • the electrons travel through the voltmeter and into the cathode • the electrons are attracted to the positively-charge metal ions in the cathode solution • the cathode ions will unite with the electrons and form a solid metal, which is deposited on the electrode – Eg. Ag+ (aq) + e- Ag (s)

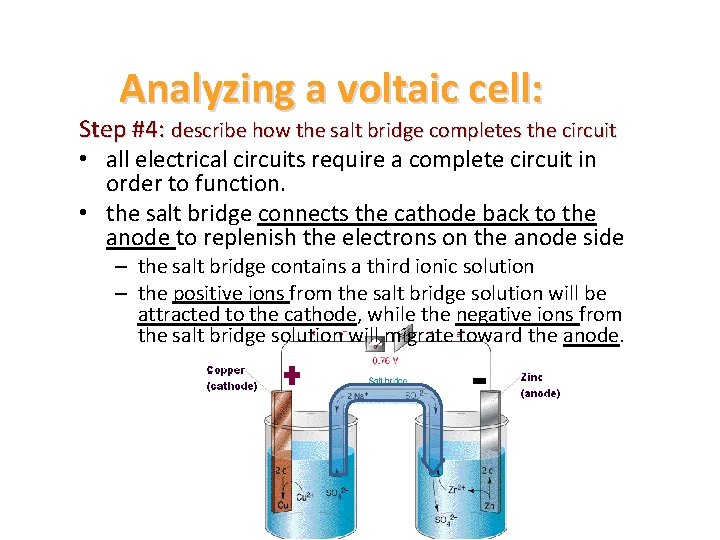
Analyzing a voltaic cell: Step #4: describe how the salt bridge completes the circuit • all electrical circuits require a complete circuit in order to function. • the salt bridge connects the cathode back to the anode to replenish the electrons on the anode side – the salt bridge contains a third ionic solution – the positive ions from the salt bridge solution will be attracted to the cathode, while the negative ions from the salt bridge solution will migrate toward the anode.

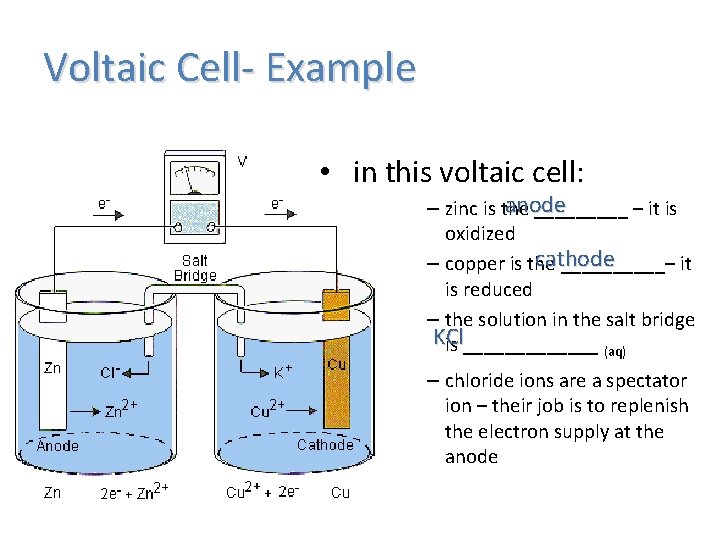
Voltaic Cell- Example • in this voltaic cell: anode – zinc is the _____ – it is oxidized cathode – copper is the _____– it is reduced – the solution in the salt bridge KCl is _______ (aq) – chloride ions are a spectator ion – their job is to replenish the electron supply at the anode

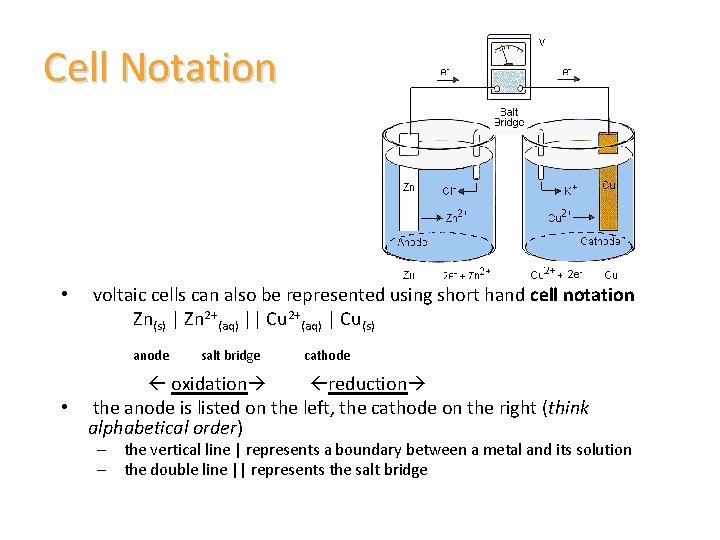
Cell Notation • voltaic cells can also be represented using short hand cell notation Zn(s) | Zn 2+(aq) || Cu 2+(aq) | Cu(s) anode salt bridge cathode oxidation reduction • the anode is listed on the left, the cathode on the right (think alphabetical order) – the vertical line | represents a boundary between a metal and its solution – the double line || represents the salt bridge

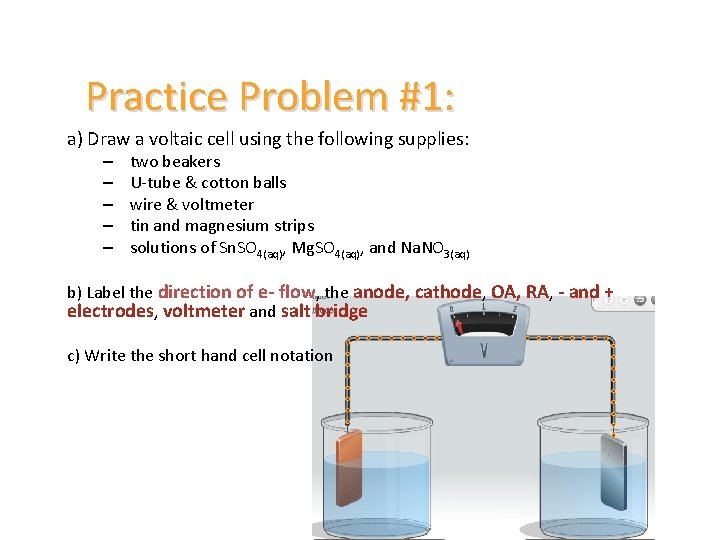
Practice Problem #1: a) Draw a voltaic cell using the following supplies: – – – two beakers U-tube & cotton balls wire & voltmeter tin and magnesium strips solutions of Sn. SO 4(aq), Mg. SO 4(aq), and Na. NO 3(aq) b) Label the direction of e- flow, the anode, cathode, OA, RA, - and + electrodes, voltmeter and salt bridge c) Write the short hand cell notation

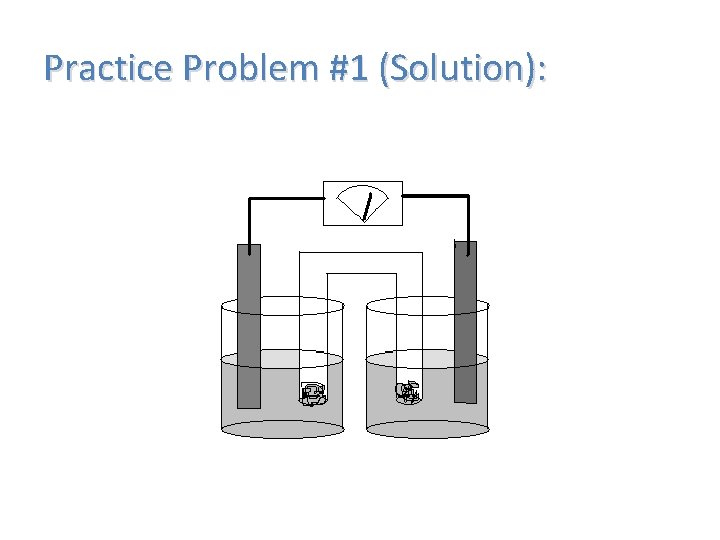
Practice Problem #1 (Solution):

Assignment: • Practice problem (page 87) – 34 • Practice problems (page 91) – 37 & 39 • Practice problem (page 92)

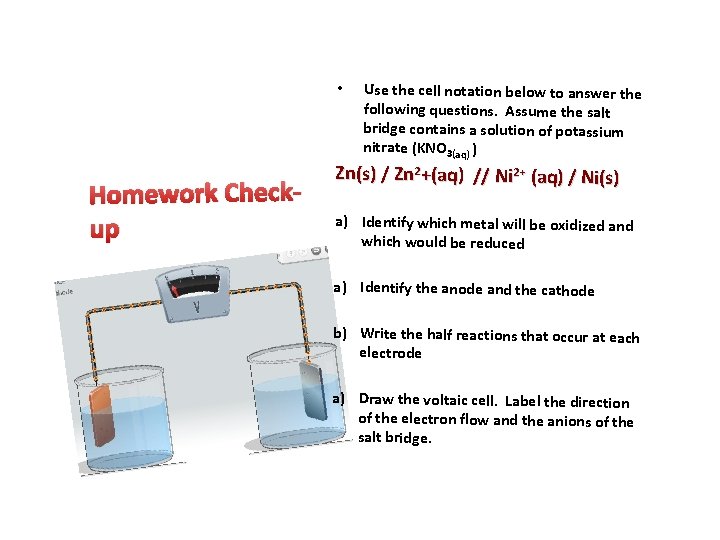
• Homework Checkup Use the cell notation below to answer the following questions. Assume the salt bridge contains a solution of potassium nitrate (KNO 3(aq) ) Zn(s) / Zn 2+(aq) // Ni 2+ (aq) / Ni(s) a) Identify which metal will be oxidized and which would be reduced a) Identify the anode and the cathode b) Write the half reactions that occur at each electrode a) Draw the voltaic cell. Label the direction of the electron flow and the anions of the salt bridge.

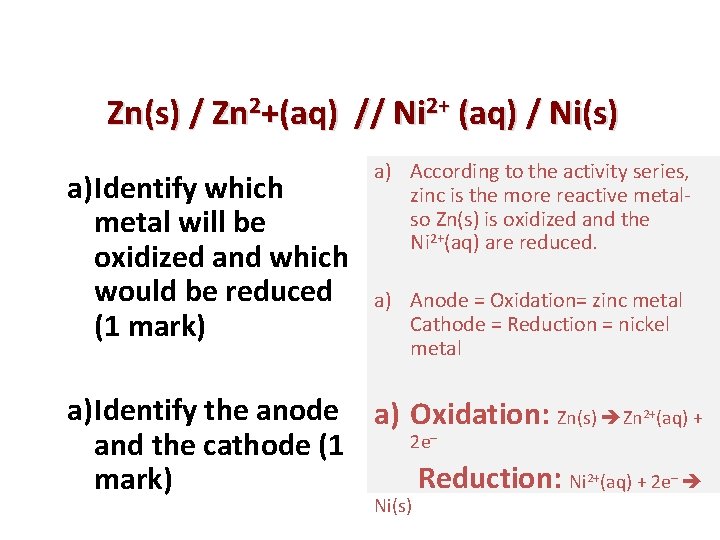
Zn(s) / Zn 2+(aq) // Ni 2+ (aq) / Ni(s) a)Identify which metal will be oxidized and which would be reduced (1 mark) a) According to the activity series, zinc is the more reactive metalso Zn(s) is oxidized and the Ni 2+(aq) are reduced. a) Anode = Oxidation= zinc metal Cathode = Reduction = nickel metal a)Identify the anode a) Oxidation: Zn(s) Zn 2+(aq) + 2 e– and the cathode (1 Reduction: Ni 2+(aq) + 2 e– mark) Ni(s)

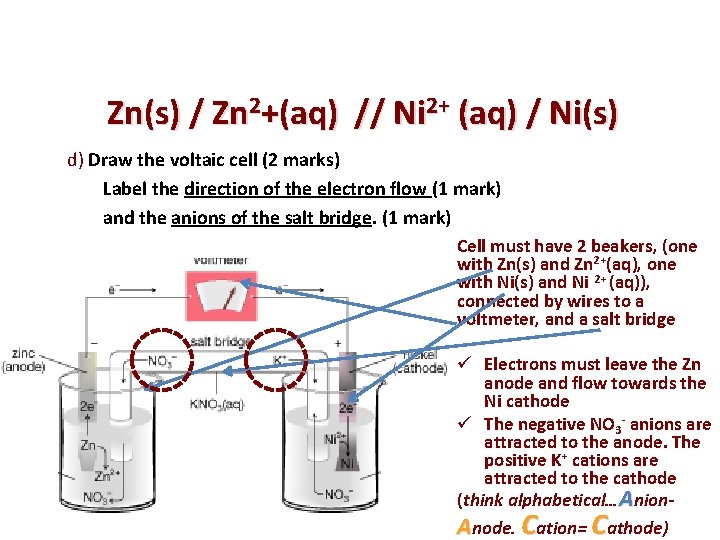
Zn(s) / Zn 2+(aq) // Ni 2+ (aq) / Ni(s) d) Draw the voltaic cell (2 marks) Label the direction of the electron flow (1 mark) and the anions of the salt bridge. (1 mark) Cell must have 2 beakers, (one with Zn(s) and Zn 2+(aq), one with Ni(s) and Ni 2+ (aq)), connected by wires to a voltmeter, and a salt bridge ü Electrons must leave the Zn anode and flow towards the Ni cathode ü The negative NO 3 - anions are attracted to the anode. The positive K+ cations are attracted to the cathode (think alphabetical…Anion. Anode. Cation= Cathode)

Assignment: ? ? • Complete the pre -lab assignment for the Voltaic Cells Lab – Your pre-lab MUST BE done, in order to participate in the

Chapter A 2 2. 5 – Electrolytic Cells

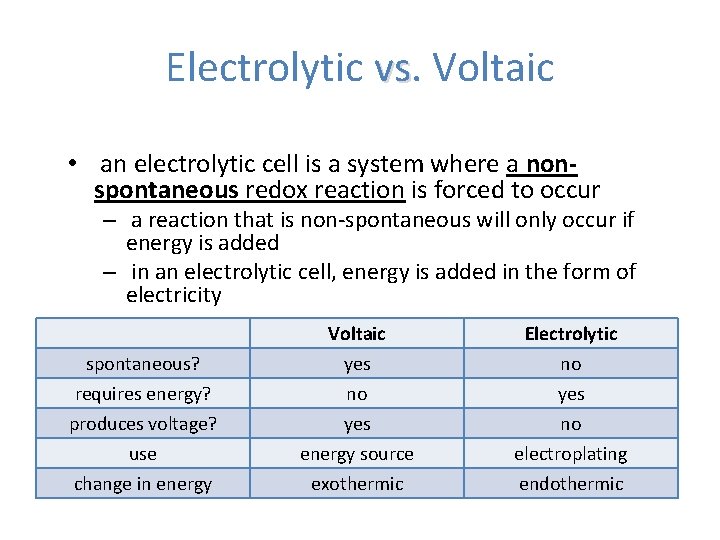
Electrolytic vs. vs Voltaic • an electrolytic cell is a system where a nonspontaneous redox reaction is forced to occur – a reaction that is non-spontaneous will only occur if energy is added – in an electrolytic cell, energy is added in the form of electricity spontaneous? requires energy? produces voltage? use change in energy Voltaic yes no yes energy source exothermic Electrolytic no yes no electroplating endothermic

Electroplating • metals, like gold and silver, that are the most stable and corrosion-resistant are also the most expensive – to manufacture a metal object that is resistant to corrosion it would NOT be cost-effective to make the whole thing out of gold – instead, a thin coating of gold is applied to the surface of a more affordable metal

Electroplating • the object to be coated is submerged in a solution of the metal ions (e. g. silver ions for objects that are to be coated in silver metal) • an external energy source (a battery) supplies energy forcing electrons to flow into the object – the negatively charged electrons will attract the positively charged metal ions from the solution, and turn them back into metal atoms, which will

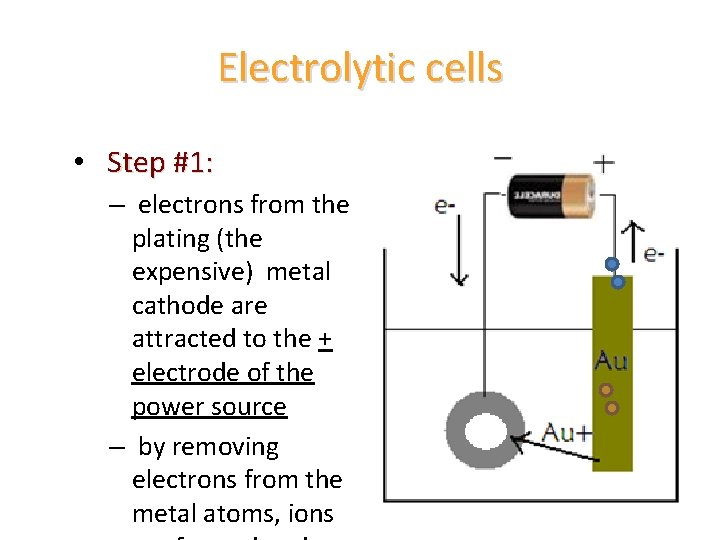
Electrolytic cells • Step #1: – electrons from the plating (the expensive) metal cathode are attracted to the + electrode of the power source – by removing electrons from the metal atoms, ions

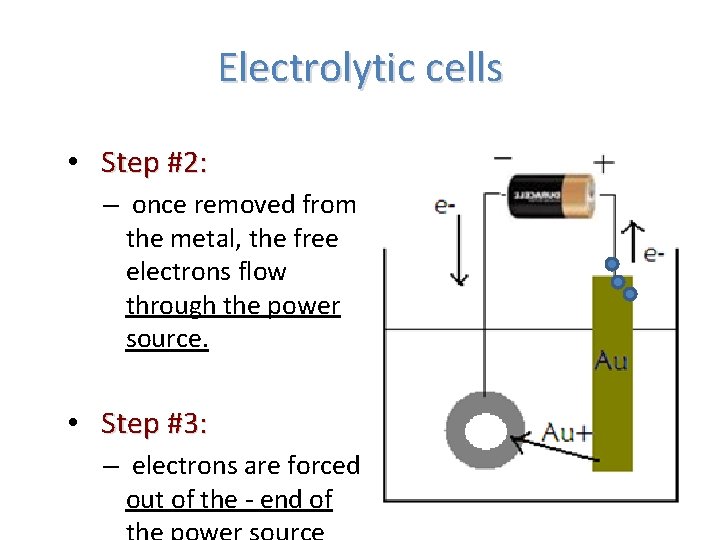
Electrolytic cells • Step #2: – once removed from the metal, the free electrons flow through the power source. • Step #3: – electrons are forced out of the - end of

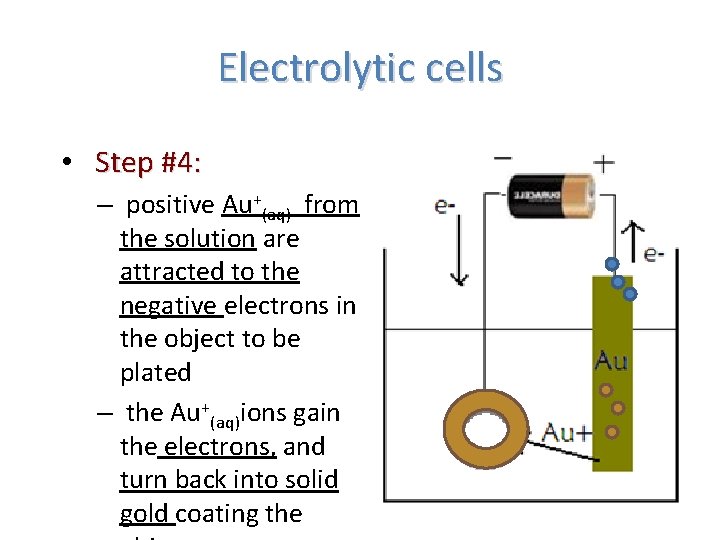
Electrolytic cells • Step #4: – positive Au+(aq) from the solution are attracted to the negative electrons in the object to be plated – the Au+(aq)ions gain the electrons, and turn back into solid gold coating the

Electroplating • electroplating is a good way to protect metals that are easily oxidized, like iron – metals that work as good electroplaters (coatings) are chromium (aka chrome), platinum, silver and gold

Gold jewelry � two types of gold jewelry exist - that which is made out of solid gold, and that which is gold plated • solid gold • gold plated – karats - pure gold is 24 K – if you have a piece – gold is a soft metal, so it of gold plated is often combined with jewelry, care must other metals like brass be taken to avoid (copper and zinc) and any deep scratches nickel to make it more • deep scratches will durable expose the – the number of karats in oxidizable metal the gold refers to how

Other uses for electrolytic cells • refining metals – a sample of impure metal (anode), pure metal (cathode) – ions of the pure metal will travel from the anode to the cathode to build up the atoms of pure metal • electrolysis – decomposition of a compound by means of an electric current – e. g. electrolysis of water makes it decompose into O 2 and H 2

Other uses for electrolytic cells • producing non-metals – non-metals, especially the halogens, are difficult to obtain in pure form because they are so reactive – non-metal atoms will accumulate around the anode of an electrolytic cell • recharging voltaic cells – when you use a battery recharger, you are using an electrolytic cell to reverse the process that occurs normally in the voltaic cell – you are literally re-charging the voltaic cell with a new supply of electrons

: t n e m n g i s As • Complete the Voltaic & Electrolytic cells Worksheet

Assignment: Prepare for your Chapter A 2 Exam