Fundamentals of Electrochemistry Introduction 1 Electrical Measurements of




- Slides: 22

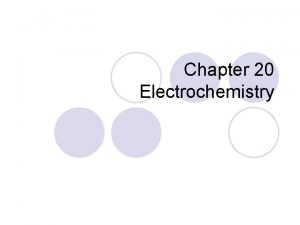
Fundamentals of Electrochemistry Introduction 1. ) Electrical Measurements of Chemical Processes Ø Redox Reaction involves transfer of electrons from one species to another. - Ø Can monitor redox reaction when electrons flow through an electric current - Ø Chemicals are separated Electric current is proportional to rate of reaction Cell voltage is proportional to free-energy change Batteries produce a direct current by converting chemical energy to electrical energy. - Common applications run the gamut from cars to ipods to laptops

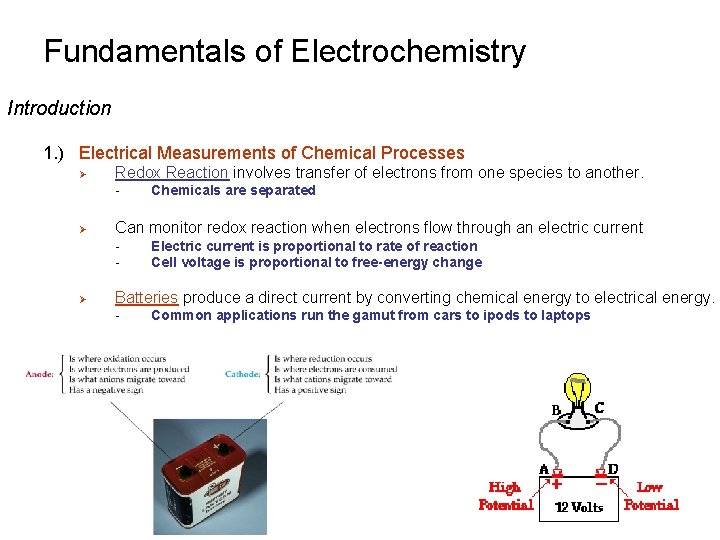
Fundamentals of Electrochemistry Basic Concepts 1. ) A Redox titration is an analytical technique based on the transfer of electrons between analyte and titrant Ø Reduction-oxidation reaction Ø A substance is reduced when it gains electrons from another substance - Ø gain of e- net decrease in charge of species Oxidizing agent (oxidant) A substance is oxidized when it loses electrons to another substance - loss of e- net increase in charge of species Reducing agent (reductant) (Reduction) (Oxidation) Oxidizing Agent Reducing Agent

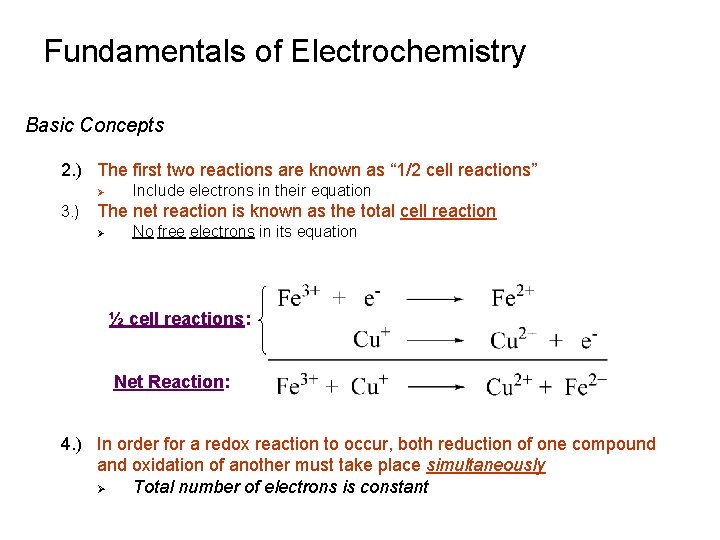
Fundamentals of Electrochemistry Basic Concepts 2. ) The first two reactions are known as “ 1/2 cell reactions” Ø 3. ) Include electrons in their equation The net reaction is known as the total cell reaction Ø No free electrons in its equation ½ cell reactions: Net Reaction: 4. ) In order for a redox reaction to occur, both reduction of one compound and oxidation of another must take place simultaneously Ø Total number of electrons is constant

Fundamentals of Electrochemistry Basic Concepts 5. ) Electric Charge (q) Ø Measured in coulombs (C) Ø Charge of a single electron is 1. 602 x 10 -19 C Ø Faraday constant (F) – 9. 649 x 104 C is the charge of a mole of electrons Relation between charge and moles: Coulombs moles 6. ) Electric current Ø Quantity of charge flowing each second through a circuit Ampere: unit of current (C/sec)

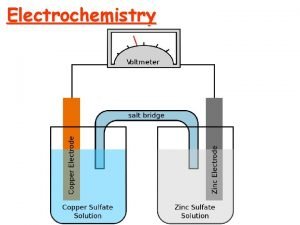
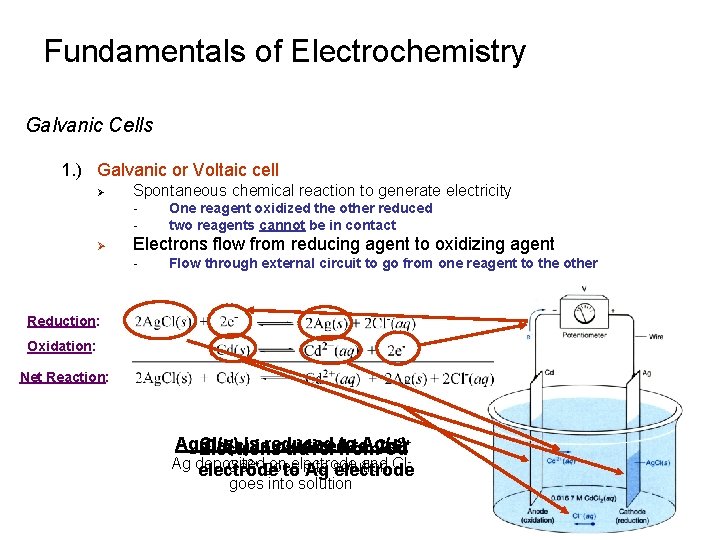
Fundamentals of Electrochemistry Galvanic Cells 1. ) Galvanic or Voltaic cell Ø Spontaneous chemical reaction to generate electricity - Ø One reagent oxidized the other reduced two reagents cannot be in contact Electrons flow from reducing agent to oxidizing agent - Flow through external circuit to go from one reagent to the other Reduction: Oxidation: Net Reaction: 2+ Ag. Cl(s) toto. Ag(s) Cd(s)isisreduced oxidized Cd Electrons travel from Cd Ag deposited onto electrode and Cl. Cd 2+ goes into electrode Ag solution electrode goes into solution

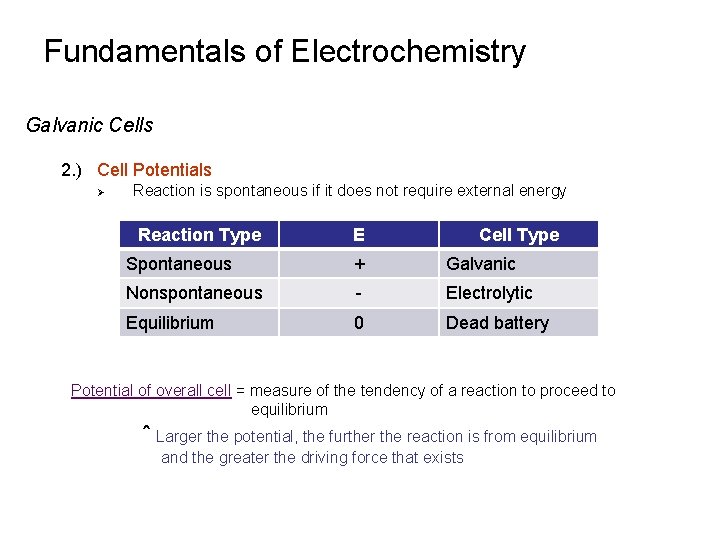
Fundamentals of Electrochemistry Galvanic Cells 2. ) Cell Potentials Ø Reaction is spontaneous if it does not require external energy Reaction Type E Cell Type Spontaneous + Galvanic Nonspontaneous - Electrolytic Equilibrium 0 Dead battery Potential of overall cell = measure of the tendency of a reaction to proceed to equilibrium ˆ Larger the potential, the further the reaction is from equilibrium and the greater the driving force that exists

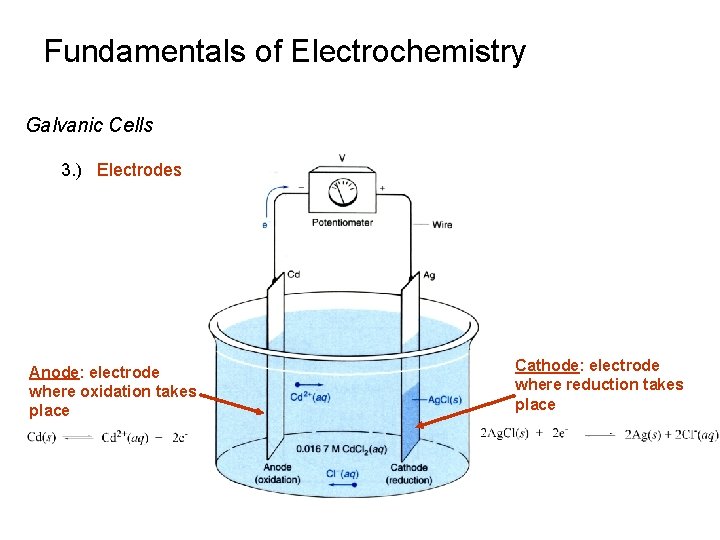
Fundamentals of Electrochemistry Galvanic Cells 3. ) Electrodes Anode: electrode where oxidation takes place Cathode: electrode where reduction takes place

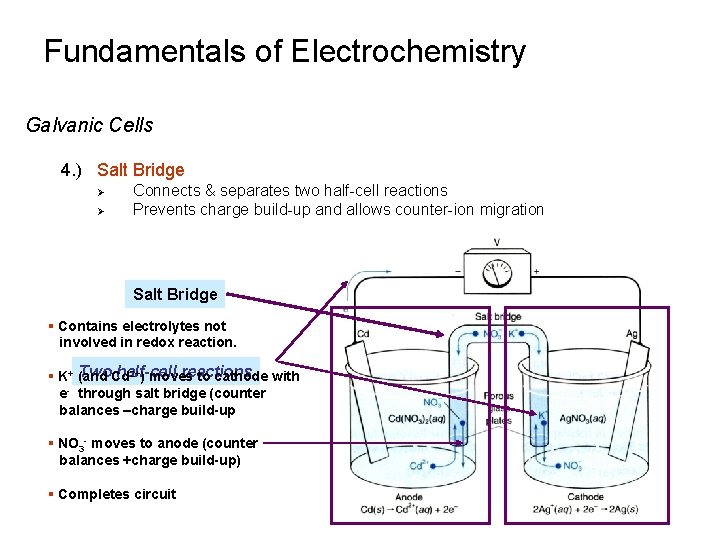
Fundamentals of Electrochemistry Galvanic Cells 4. ) Salt Bridge Ø Ø Connects & separates two half-cell reactions Prevents charge build-up and allows counter-ion migration Salt Bridge § Contains electrolytes not involved in redox reaction. Two. Cd half-cell reactions 2+) moves § K+ (and to cathode with e through salt bridge (counter balances –charge build-up § NO 3 - moves to anode (counter balances +charge build-up) § Completes circuit

Fundamentals of Electrochemistry Galvanic Cells 5. ) Short-Hand Notation Ø Representation of Cells: by convention start with anode on left Phase boundary Electrode/solution interface anode Zn|Zn. SO 4(a. ZN 2+ = 0. 0100)||Cu. SO 4(a. Cu 2+ = 0. 0100)|Cu Solution in contact with anode & its concentration 2 liquid junctions due to salt bridge cathode Solution in contact with cathode & its concentration

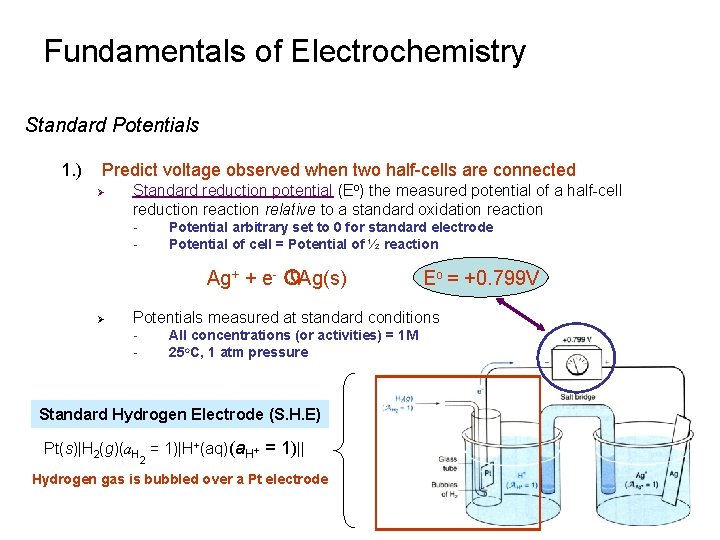
Fundamentals of Electrochemistry Standard Potentials 1. ) Predict voltage observed when two half-cells are connected Ø Standard reduction potential (Eo) the measured potential of a half-cell reduction reaction relative to a standard oxidation reaction - Potential arbitrary set to 0 for standard electrode Potential of cell = Potential of ½ reaction Ag+ + e- » Ag(s) Ø Eo = +0. 799 V Potentials measured at standard conditions - All concentrations (or activities) = 1 M 25 o. C, 1 atm pressure Standard Hydrogen Electrode (S. H. E) Pt(s)|H 2(g)(a. H = 1)|H+(aq)(a. H+ = 1)|| 2 Hydrogen gas is bubbled over a Pt electrode

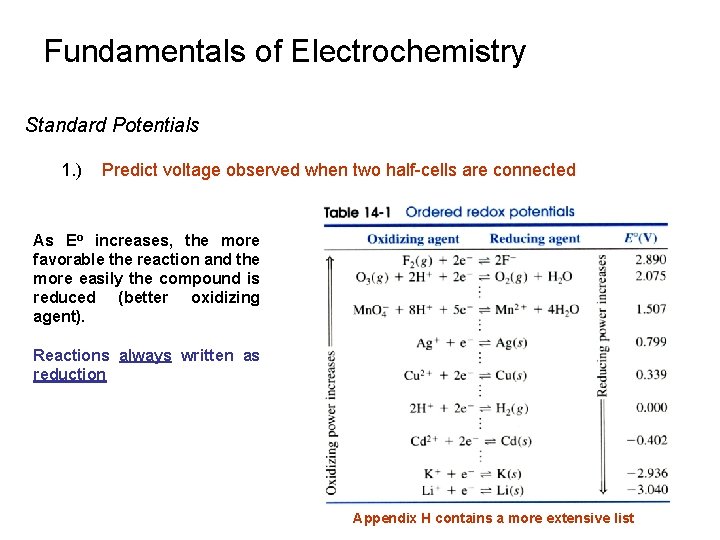
Fundamentals of Electrochemistry Standard Potentials 1. ) Predict voltage observed when two half-cells are connected As Eo increases, the more favorable the reaction and the more easily the compound is reduced (better oxidizing agent). Reactions always written as reduction Appendix H contains a more extensive list

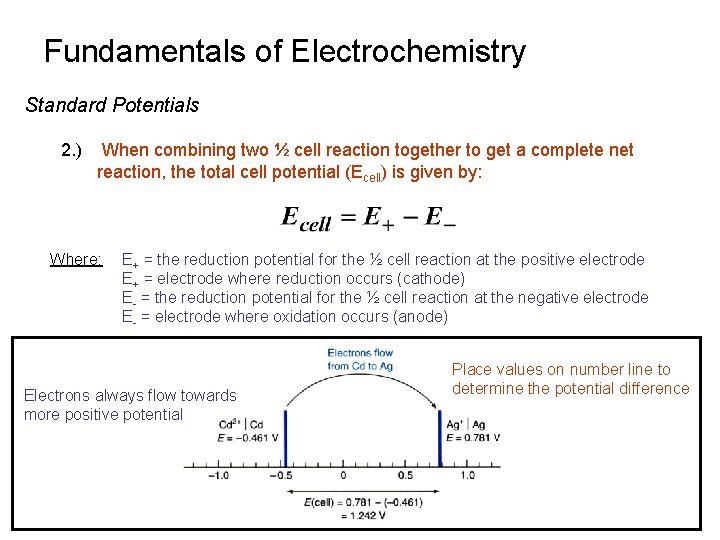
Fundamentals of Electrochemistry Standard Potentials 2. ) When combining two ½ cell reaction together to get a complete net reaction, the total cell potential (Ecell) is given by: Where: E+ = the reduction potential for the ½ cell reaction at the positive electrode E+ = electrode where reduction occurs (cathode) E- = the reduction potential for the ½ cell reaction at the negative electrode E- = electrode where oxidation occurs (anode) Electrons always flow towards more positive potential Place values on number line to determine the potential difference

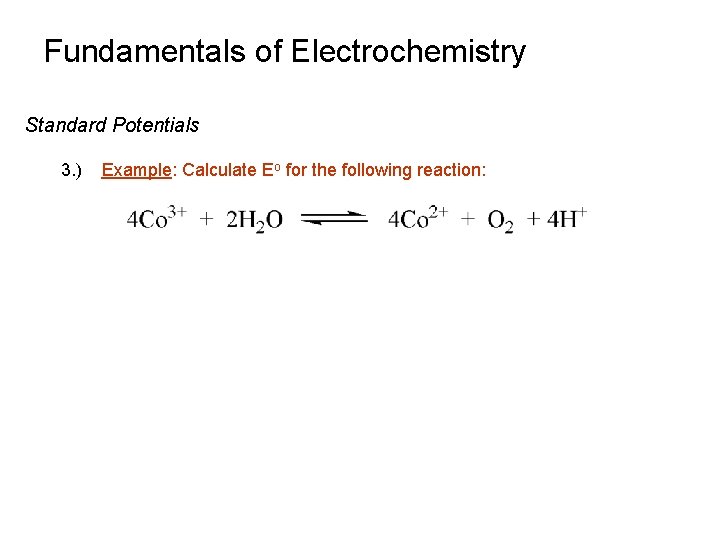
Fundamentals of Electrochemistry Standard Potentials 3. ) Example: Calculate Eo for the following reaction:

Fundamentals of Electrochemistry Nernst Equation 1. ) Reduction Potential under Non-standard Conditions Ø Ø E determined using Nernst Equation Concentrations not-equal to 1 M For the given reaction: a. A + ne- » b. B Eo The ½ cell reduction potential is given by: Where: at 25 o. C E = actual ½ cell reduction potential Eo = standard ½ cell reduction potential n = number of electrons in reaction T = temperature (K) R = ideal gas law constant (8. 314 J/(K-mol) F = Faraday’s constant (9. 649 x 104 C/mol) A = activity of A or B

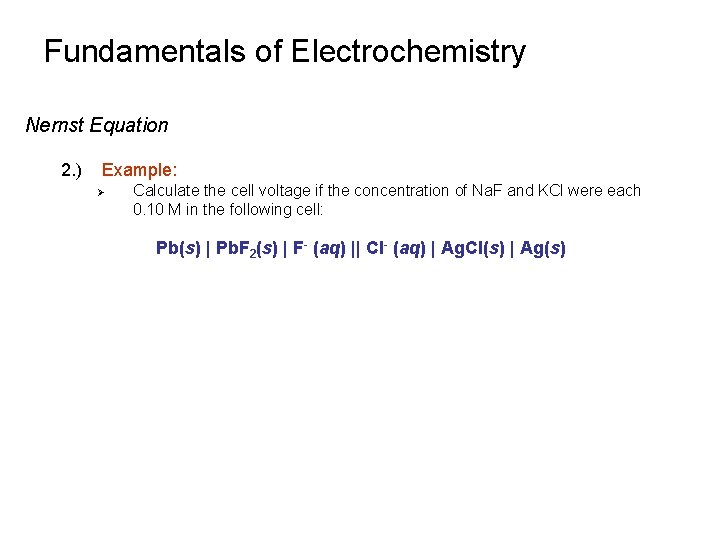
Fundamentals of Electrochemistry Nernst Equation 2. ) Example: Ø Calculate the cell voltage if the concentration of Na. F and KCl were each 0. 10 M in the following cell: Pb(s) | Pb. F 2(s) | F- (aq) || Cl- (aq) | Ag. Cl(s) | Ag(s)

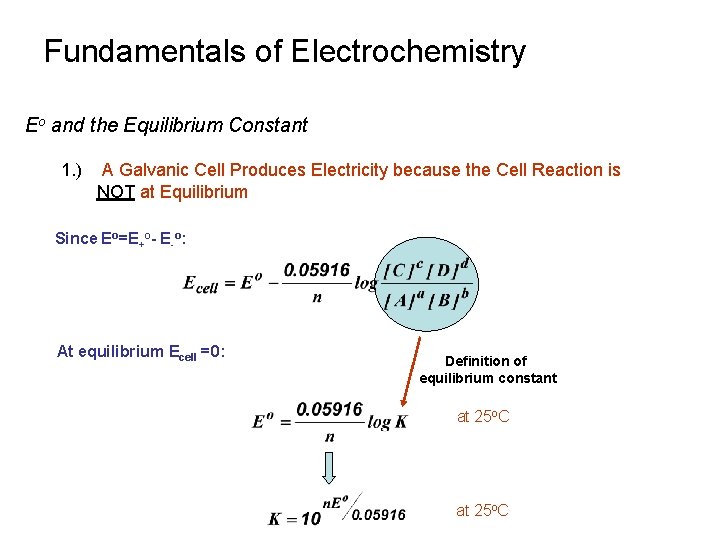
Fundamentals of Electrochemistry Eo and the Equilibrium Constant 1. ) A Galvanic Cell Produces Electricity because the Cell Reaction is NOT at Equilibrium Ø Ø Concentration in two cells change with current Concentration will continue to change until Equilibrium is reached E = 0 V at equilibrium Battery is “dead” Consider the following ½ cell reactions: a. A + ne- » c. C d. D + ne- » b. B Cell potential in terms of Nernst Equation is: Simplify: E+ o E- o

Fundamentals of Electrochemistry Eo and the Equilibrium Constant 1. ) A Galvanic Cell Produces Electricity because the Cell Reaction is NOT at Equilibrium Since Eo=E+o- E-o: At equilibrium Ecell =0: Definition of equilibrium constant at 25 o. C

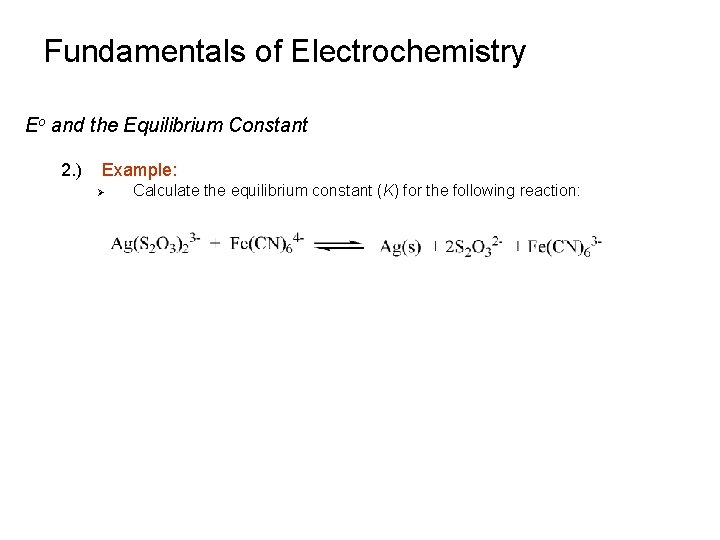
Fundamentals of Electrochemistry Eo and the Equilibrium Constant 2. ) Example: Ø Calculate the equilibrium constant (K) for the following reaction:

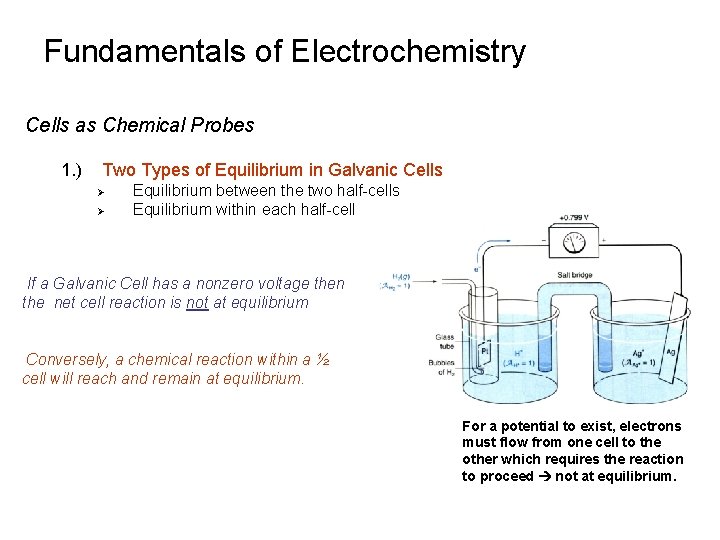
Fundamentals of Electrochemistry Cells as Chemical Probes 1. ) Two Types of Equilibrium in Galvanic Cells Ø Ø Equilibrium between the two half-cells Equilibrium within each half-cell If a Galvanic Cell has a nonzero voltage then the net cell reaction is not at equilibrium Conversely, a chemical reaction within a ½ cell will reach and remain at equilibrium. For a potential to exist, electrons must flow from one cell to the other which requires the reaction to proceed not at equilibrium.

Fundamentals of Electrochemistry Cells as Chemical Probes 2. ) Example: Ø If the voltage for the following cell is 0. 512 V, find Ksp for Cu(IO 3)2: Ni(s)|Ni. SO 4(0. 0025 M)||KIO 3(0. 10 M)|Cu(IO 3)2(s)|Cu(s)

Fundamentals of Electrochemistry Biochemists Use Eo´ 1. ) Redox Potentials Containing Acids or Bases are p. H Dependent Ø Ø Standard potential all concentrations = 1 M p. H=0 for [H+] = 1 M 2. ) p. H Inside of a Plant or Animal Cell is ~ 7 Ø Standard potentials at p. H =0 not appropriate for biological systems - Reduction or oxidation strength may be reversed at p. H 0 compared to p. H 7 Metabolic Pathways

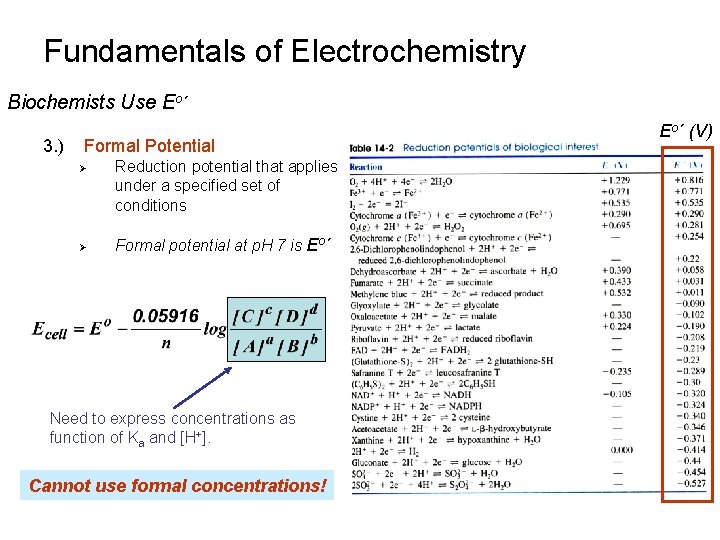
Fundamentals of Electrochemistry Biochemists Use Eo´ 3. ) Formal Potential Ø Ø Reduction potential that applies under a specified set of conditions Formal potential at p. H 7 is Eo´ Need to express concentrations as function of Ka and [H+]. Cannot use formal concentrations! Eo´ (V)
 Fundamentals of electrochemistry
Fundamentals of electrochemistry Fundamentals of electrochemistry
Fundamentals of electrochemistry Intro to electrochemistry
Intro to electrochemistry Electroanalytical chemistry
Electroanalytical chemistry Ohm's law
Ohm's law Chapter 39 electrical fundamentals
Chapter 39 electrical fundamentals Electrical engineering fundamentals 66712 pdf
Electrical engineering fundamentals 66712 pdf Joints
Joints Basic chemistry tutorial
Basic chemistry tutorial Transport number
Transport number Junction potential
Junction potential Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Electrochemistry
Electrochemistry What is electrochemistry
What is electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Electrochemistry stoichiometry
Electrochemistry stoichiometry Balancing redox reactions khan academy
Balancing redox reactions khan academy Basic electrochemistry
Basic electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Ap chem electrochemistry review
Ap chem electrochemistry review Ap chemistry electrochemistry
Ap chemistry electrochemistry Redox reaction
Redox reaction Chapter 21 electrochemistry
Chapter 21 electrochemistry