Voltaic or Galvanic Cells In a voltaic or

- Slides: 8

Voltaic (or Galvanic) Cells In a voltaic (or galvanic) cell, e– transfer occurs via an external pathway that links the reactants. e. g. , a battery an or ex ic ox electrodes: the two solid metals in a voltaic/galvanic cell fat, red anode cathode cat -- oxidation occurs here -- reduction occurs here -- written w/a (–) sign -- written w/a (+) sign

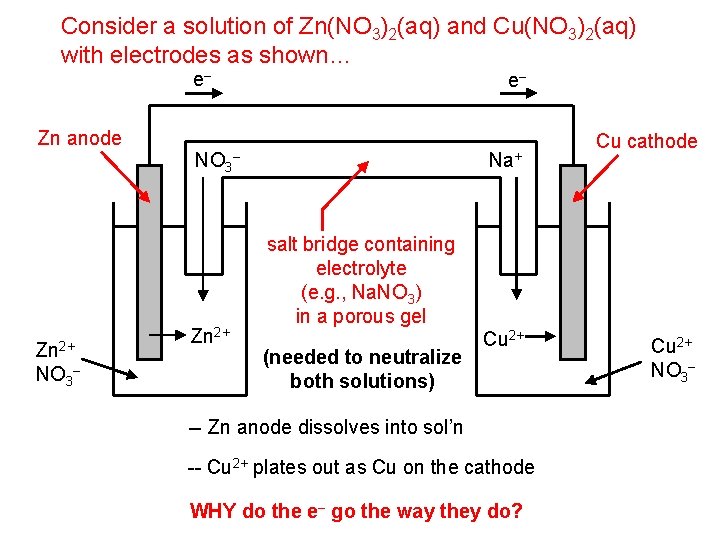
Consider a solution of Zn(NO 3)2(aq) and Cu(NO 3)2(aq) with electrodes as shown… e– Zn anode Zn 2+ NO 3 – NO 3 Zn 2+ e– Na+ – Cu cathode salt bridge containing electrolyte (e. g. , Na. NO 3) in a porous gel (needed to neutralize both solutions) Cu 2+ -- Zn anode dissolves into sol’n -- Cu 2+ plates out as Cu on the cathode WHY do the e– go the way they do? Cu 2+ NO 3–

Cell EMF PE of anode’s e– > PE of cathode’s e– Thus… anode’s e–s spontaneously flow towards cathode, if given a path. PE is important; charge # of charges is NOT. Difference in Voltage (or “potential”) difference is also called the electromotive force (emf).

For a particular cell, (i. e. , a particular anode and cathode), the cell’s emf is written Ecell and is called the cell potential. -- it is + for spontaneous cell reactions -- it depends on materials, conc. , and temp. -- standard emfs occur at 25 o. C -- To calculate Ecell, look up tabulated standard reduction potentials for each half-cell… e. g. , Ag+(aq) + e– Ag(s) Eored = +0. 80 V …and then use the equation: Eocell = Eored, cath – Eored, an (Look it up!)

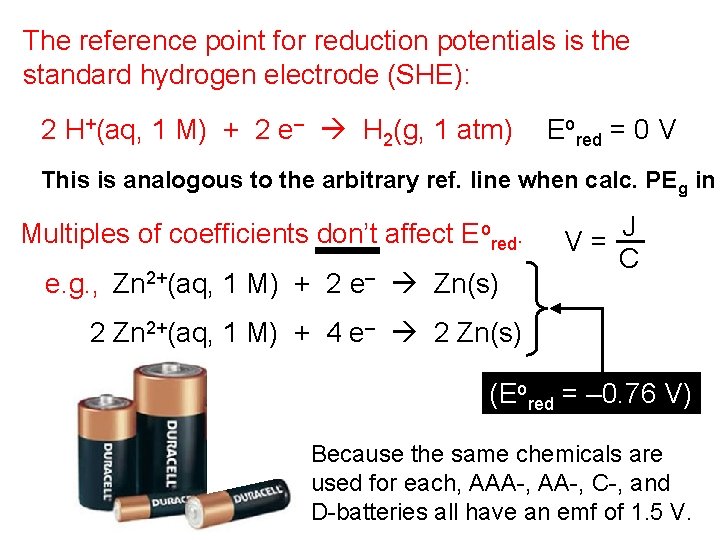
The reference point for reduction potentials is the standard hydrogen electrode (SHE): 2 H+(aq, 1 M) + 2 e– H 2(g, 1 atm) Eored = 0 V This is analogous to the arbitrary ref. line when calc. PE g in Multiples of coefficients don’t affect Eored. e. g. , Zn 2+(aq, 1 M) + 2 e– Zn(s) V= J C 2 Zn 2+(aq, 1 M) + 4 e– 2 Zn(s) (Eored = – 0. 76 V) Because the same chemicals are used for each, AAA-, C-, and D-batteries all have an emf of 1. 5 V.

A 9 -volt battery is six AAA batteries wired together in series.

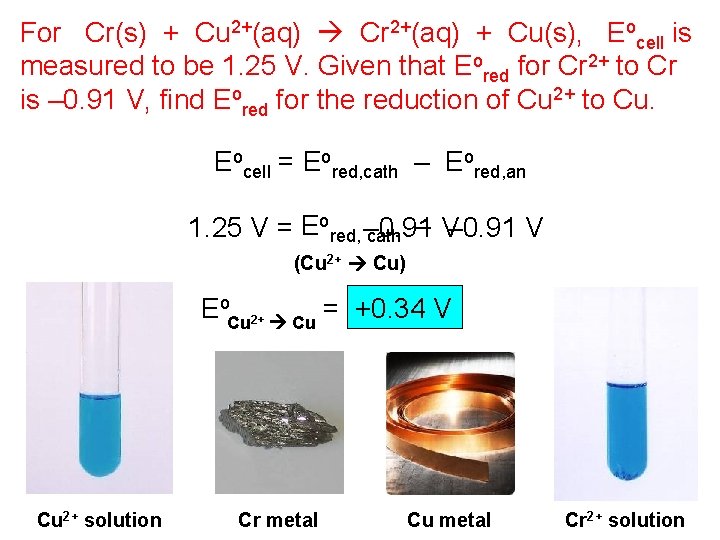
For Cr(s) + Cu 2+(aq) Cr 2+(aq) + Cu(s), Eocell is measured to be 1. 25 V. Given that Eored for Cr 2+ to Cr is – 0. 91 V, find Eored for the reduction of Cu 2+ to Cu. Eocell = Eored, cath – Eored, an 1. 25 V = Eored, – 0. 91 V cath – V (Cu 2+ Cu) Eo. Cu 2+ Cu = +0. 34 V Cu 2+ solution Cr metal Cu metal Cr 2+ solution

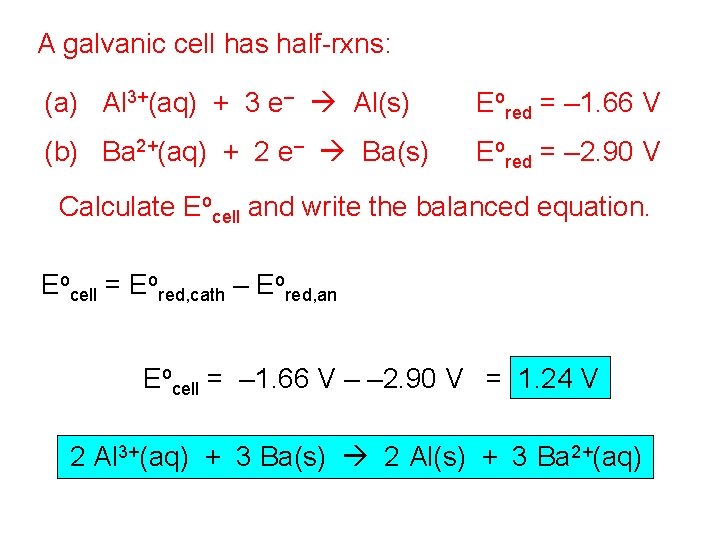
A galvanic cell has half-rxns: (a) Al 3+(aq) + 3 e– Al(s) Eored = – 1. 66 V (b) Ba 2+(aq) + 2 e– Ba(s) Eored = – 2. 90 V Calculate Eocell and write the balanced equation. Eocell = Eored, cath – Eored, an For a galvanic cell, Eo must be > 0. Thus, (a) represents the cathode and (b) represents the anode. Eocell = – 1. 66 V – – 2. 90 V = 1. 24 V 2 Al 3+(aq) + 3 Ba(s) 2 Al(s) + 3 Ba 2+(aq)