Section 1 Voltaic Cells In voltaic cells oxidation

- Slides: 16

Section 1: Voltaic Cells In voltaic cells, oxidation takes place at the anode, yielding electrons that flow to the cathode, where reduction occurs. K What I Know W What I Want to Find Out L What I Learned

• 10(H) Understand differentiate among acid-base reactions, precipitation reactions, and oxidation-reduction reactions. • 8(D) Use the law of conservation of mass to write and balance chemical equations. Copyright © Mc. Graw-Hill Education Voltaic Cells

Essential Questions • How is electrical energy obtained from a redox reaction? • What are the parts of a voltaic cell, and how does each part operate? Copyright © Mc. Graw-Hill Education Voltaic Cells

Vocabulary Review New • • • oxidation reduction Copyright © Mc. Graw-Hill Education salt bridge electrochemical cell voltaic cell half-cell anode cathode reduction potential standard hydrogen electrode Voltaic Cells

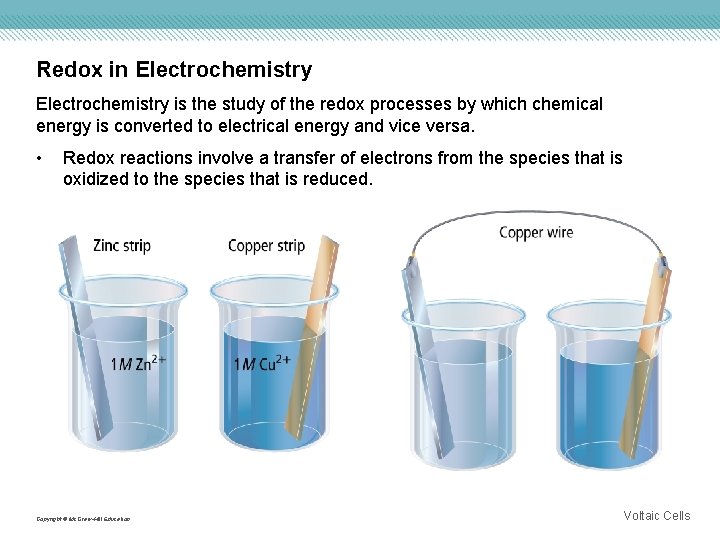
Redox in Electrochemistry is the study of the redox processes by which chemical energy is converted to electrical energy and vice versa. • Redox reactions involve a transfer of electrons from the species that is oxidized to the species that is reduced. Copyright © Mc. Graw-Hill Education Voltaic Cells

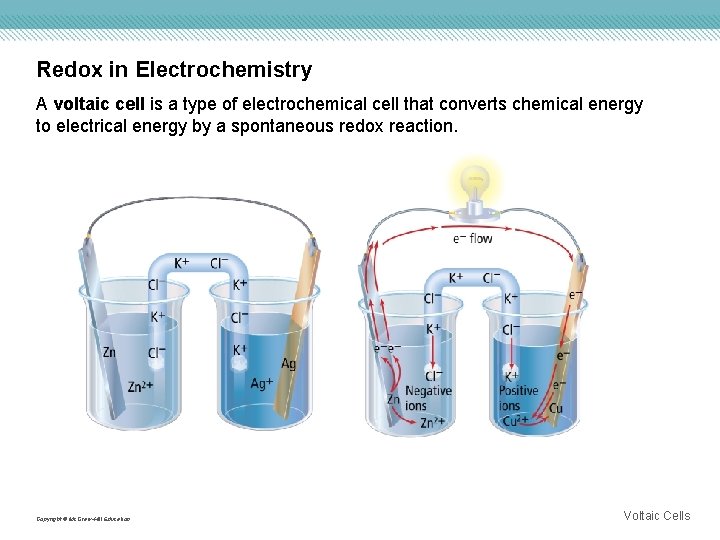
Redox in Electrochemistry A salt bridge is a pathway to allow the passage of ions from one side to another, so that ions do not build up around the electrodes. An electrochemical cell is an apparatus that uses a redox reaction to produce electrical energy or uses electrical energy to cause a chemical reaction. Copyright © Mc. Graw-Hill Education Voltaic Cells

Redox in Electrochemistry A voltaic cell is a type of electrochemical cell that converts chemical energy to electrical energy by a spontaneous redox reaction. Copyright © Mc. Graw-Hill Education Voltaic Cells

Voltaic Cell Animation VOLTAIC CELL Add link to concepts in motion animation from page 709 here. Copyright © Mc. Graw-Hill Education Voltaic Cells

Chemistry of Voltaic Cells An electrochemical cell consists of two parts called half-cells, in which the separate oxidation and reduction reactions take place. • The electrode where oxidation takes place is called the anode. • The cathode is the electrode where reduction occurs. • Electric potential energy is a measure of the amount of current that can be generated from a voltaic cell to do work. • Electric charge can flow between two points only when a difference in electric potential energy exists between the two points. • A volt is a unit used to measure cell potential—the force from the difference in electric potential energy between two electrodes. Copyright © Mc. Graw-Hill Education Voltaic Cells

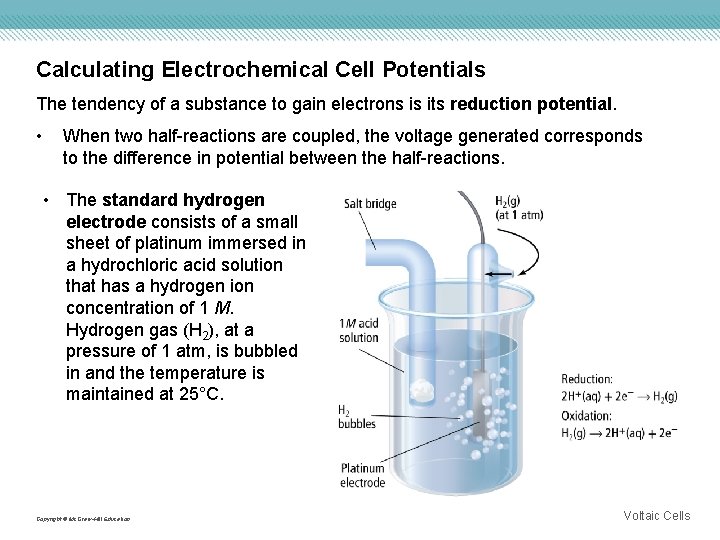
Calculating Electrochemical Cell Potentials The tendency of a substance to gain electrons is its reduction potential. • When two half-reactions are coupled, the voltage generated corresponds to the difference in potential between the half-reactions. • The standard hydrogen electrode consists of a small sheet of platinum immersed in a hydrochloric acid solution that has a hydrogen ion concentration of 1 M. Hydrogen gas (H 2), at a pressure of 1 atm, is bubbled in and the temperature is maintained at 25°C. Copyright © Mc. Graw-Hill Education Voltaic Cells

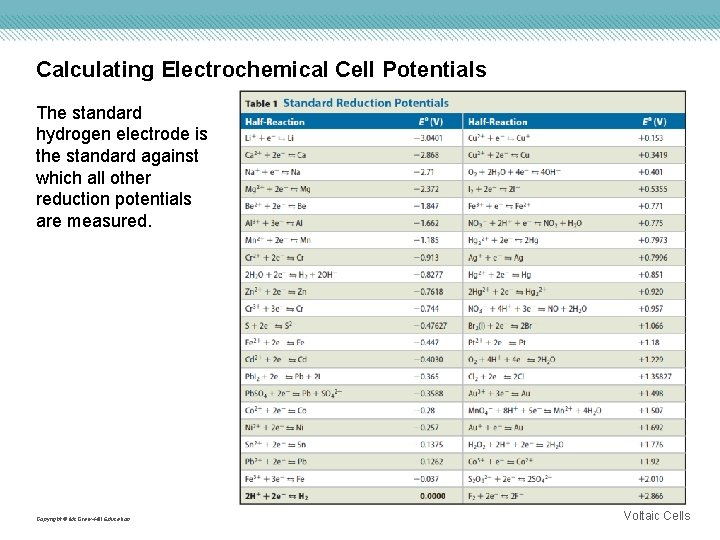
Calculating Electrochemical Cell Potentials The standard hydrogen electrode is the standard against which all other reduction potentials are measured. Copyright © Mc. Graw-Hill Education Voltaic Cells

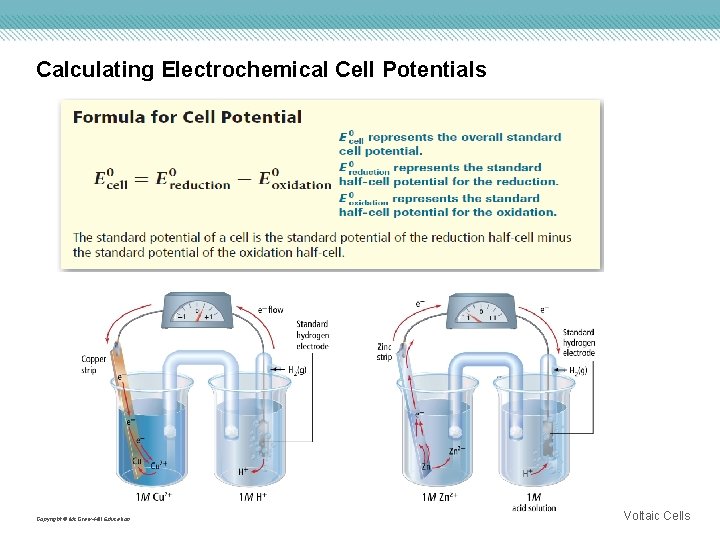
Calculating Electrochemical Cell Potentials Copyright © Mc. Graw-Hill Education Voltaic Cells

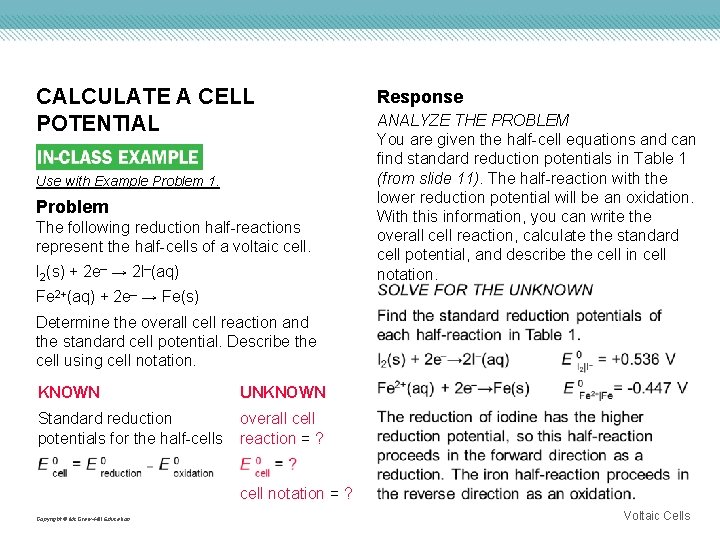
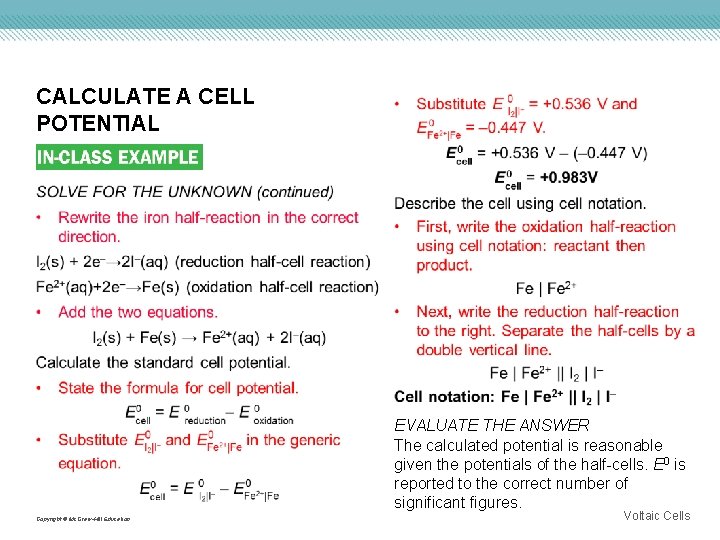
CALCULATE A CELL POTENTIAL Use with Example Problem 1. Problem The following reduction half-reactions represent the half-cells of a voltaic cell. I 2(s) + 2 e– → 2 I–(aq) Response ANALYZE THE PROBLEM You are given the half-cell equations and can find standard reduction potentials in Table 1 (from slide 11). The half-reaction with the lower reduction potential will be an oxidation. With this information, you can write the overall cell reaction, calculate the standard cell potential, and describe the cell in cell notation. Fe 2+(aq) + 2 e– → Fe(s) Determine the overall cell reaction and the standard cell potential. Describe the cell using cell notation. KNOWN UNKNOWN Standard reduction potentials for the half-cells overall cell reaction = ? cell notation = ? Copyright © Mc. Graw-Hill Education Voltaic Cells

CALCULATE A CELL POTENTIAL EVALUATE THE ANSWER The calculated potential is reasonable given the potentials of the half-cells. E 0 is reported to the correct number of significant figures. Copyright © Mc. Graw-Hill Education Voltaic Cells

Use Standard Reduction Potentials • Cell potentials can be used to determine if a proposed reaction under standard conditions will be spontaneous. • If the calculated potential is positive, the reaction is spontaneous. • If the calculated potential is negative, the reaction is not spontaneous. Copyright © Mc. Graw-Hill Education Voltaic Cells

Review Essential Questions • How is electrical energy obtained from a redox reaction? • What are the parts of a voltaic cell, and how does each part operate? Vocabulary • salt bridge • electrochemical cell • voltaic cell Copyright © Mc. Graw-Hill Education • half-cell • anode • cathode • reduction potential • standard hydrogen electrode Voltaic Cells