Electrochemistry Electrochemical Cells Chemical Cells Also called voltaic












- Slides: 19

Electrochemistry

Electrochemical Cells/ Chemical Cells § § § Also called voltaic or galvanic cells A redox reaction produces electricity Occurs spontaneously

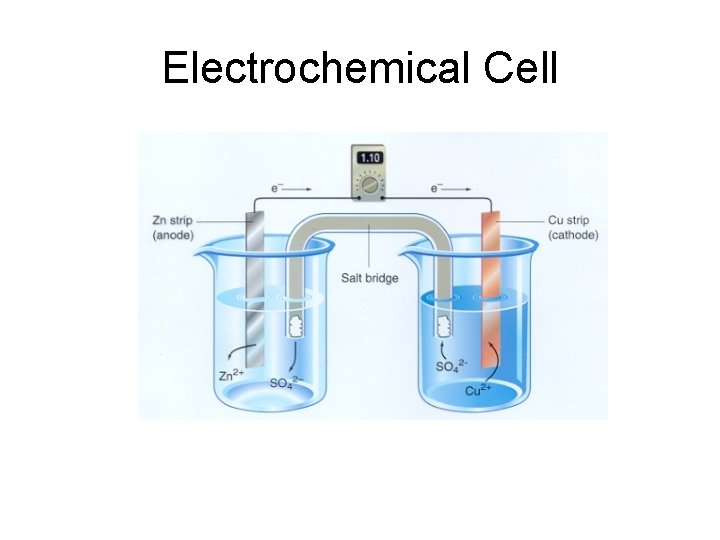
Electrochemical Cell

Half Cells Each ½ of the redox reaction occurs in a separate container – One for oxidation and one for reduction They are connected by a salt bridge – Salt Bridge: allows ions to flow between the two cells

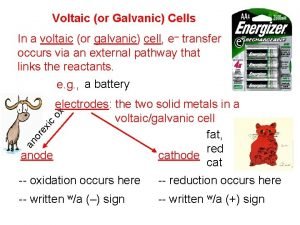
Electrodes Metals which provide a surface for oxidation or reduction to occur – Solids – Oxidation Number = 0 – Anode – Cathode

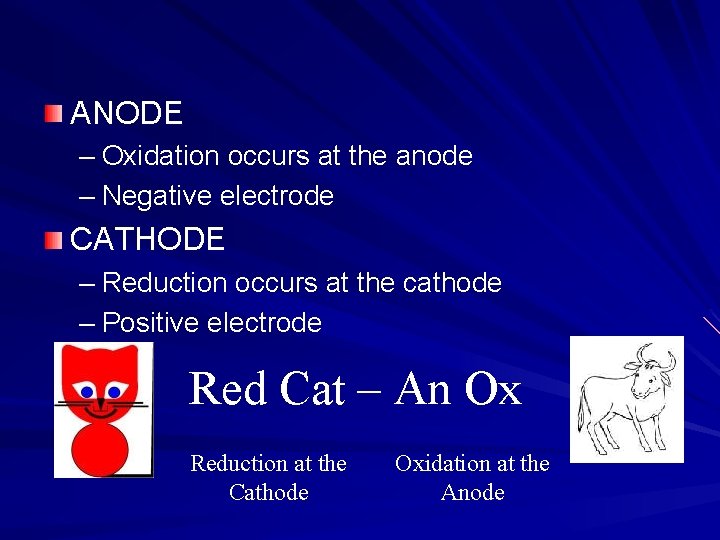
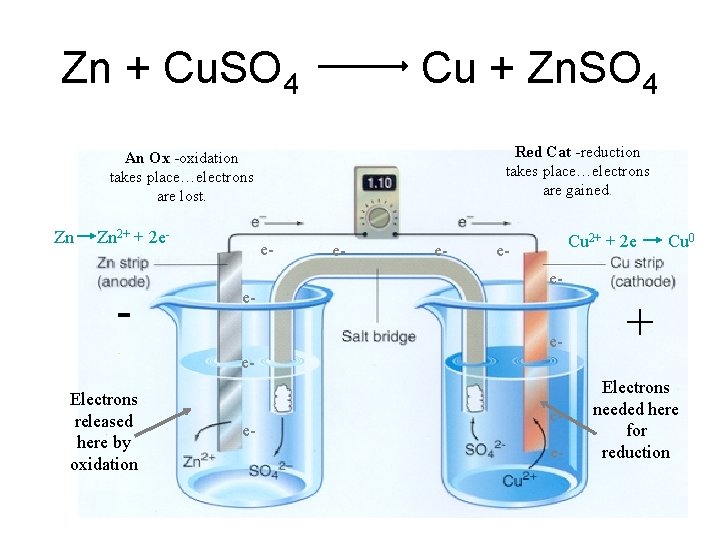
ANODE – Oxidation occurs at the anode – Negative electrode CATHODE – Reduction occurs at the cathode – Positive electrode Red Cat – An Ox Reduction at the Cathode Oxidation at the Anode

Flow of Electrons The electrodes are connected by a wire Electrons flow from the anode to the cathode through the wire

Why does the cell produce electricity? There is a difference of electric potential between the two electrodes – Electrons will flow between the two electrodes until equilibrium is reached – At equilibrium the cell’s voltage would be zero

Zn + Cu. SO 4 Cu + Zn. SO 4 Red Cat -reduction takes place…electrons are gained. An Ox -oxidation takes place…electrons are lost. Zn Zn 2+ + 2 e- - ee- e- e- Cu 2+ + 2 e e- - Cu 0 ee- + e. Electrons released here by oxidation e- ee- Electrons needed here for reduction

Batteries • Use a redox reaction which produces electricity spontaneously • Batteries are recharged by reversing the reaction • Dry Cell (Acid or Alkaline), Lead Storage (Car), Rechargeable (Ni/Cd)

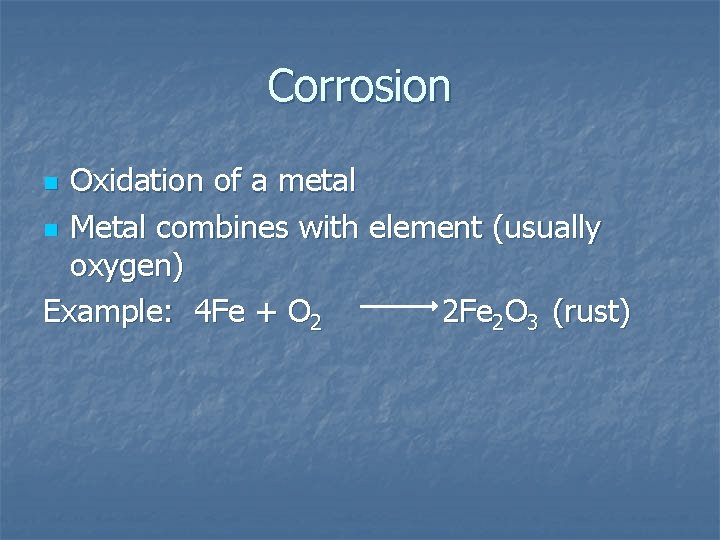
Corrosion Oxidation of a metal n Metal combines with element (usually oxygen) Example: 4 Fe + O 2 2 Fe 2 O 3 (rust) n

Prevention of Rust n n Cover the metal – paint, oil, another (more reactive) metal Cathodic Prevention metal is placed in contact with a more reactive metal n That metal will be oxidized (acts as the anode), the original metal acts as the cathode n n Alloys – mixture of metals n Brass, stainless steel (Fe + Cr), cast iron (C + Si)

Electrolytic Cells u Also called electrolysis u An electric current is used to produce a chemical reaction – An electric current is used to force a non -spontaneous reaction to occur

u Oxidation occurs at the anode u Reduction occurs at the cathode u Electrons flow from anode to cathode u The cathode is the negative electrode u The anode is the positive electrode – This is opposite of the chemical cell because the external current causes the polarities to switch


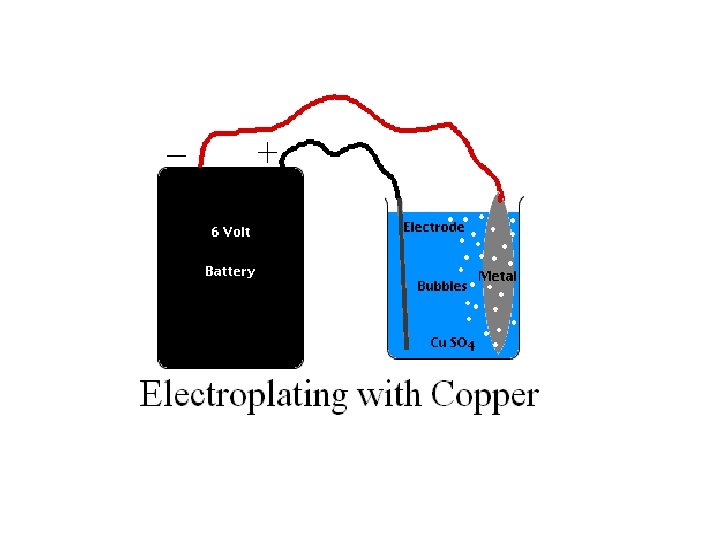
Electroplating Object to be plated is the CATHODE, negative n Metal to be plated onto the object is the ANODE, positive n Solution must contain ions of the metal to be plated n

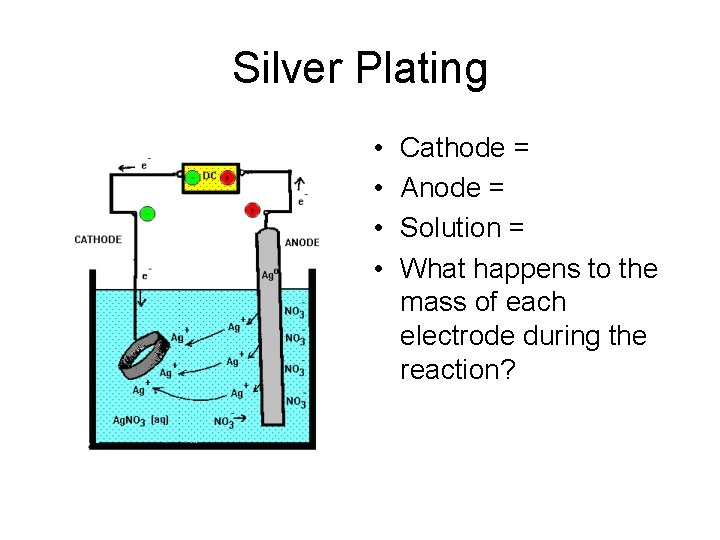
Silver Plating • • Cathode = Anode = Solution = What happens to the mass of each electrode during the reaction?

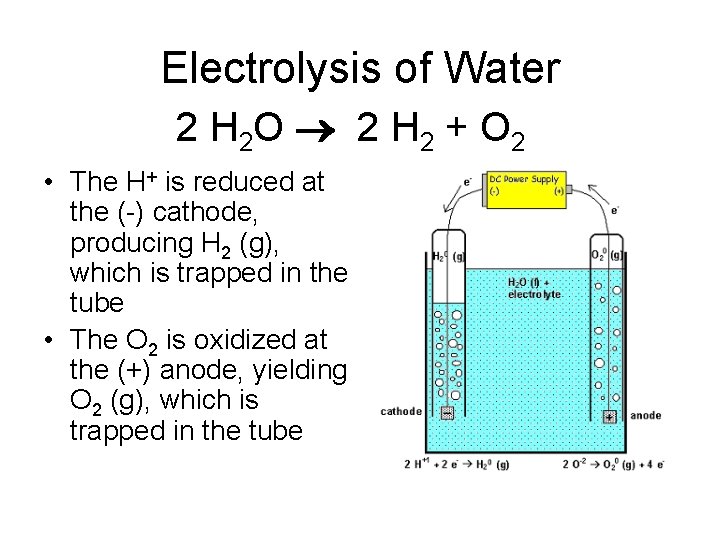
Electrolysis of Water 2 H 2 O 2 H 2 + O 2 • The H+ is reduced at the (-) cathode, producing H 2 (g), which is trapped in the tube • The O 2 is oxidized at the (+) anode, yielding O 2 (g), which is trapped in the tube

Hydrogen Fuel Cells • Uses hydrogen gas as the fuel 2 H 2 + O 2 2 H 2 O
 Voltaic cells example #2 worksheet answers
Voltaic cells example #2 worksheet answers Electrolytic cell
Electrolytic cell Voltaic and galvanic cells
Voltaic and galvanic cells Body cells are also called
Body cells are also called Balancing redox reactions
Balancing redox reactions Voltaic cell electron flow
Voltaic cell electron flow Lead acid battery primary or secondary
Lead acid battery primary or secondary Voltaic cell virtual lab
Voltaic cell virtual lab Voltaic fundamental
Voltaic fundamental Voltaic fundamentals
Voltaic fundamentals Voltaic fundamentals
Voltaic fundamentals Voltaic aim
Voltaic aim Red cat electrochemistry
Red cat electrochemistry Cell chapter 20
Cell chapter 20 Electrochemical deposition
Electrochemical deposition Waterline corrosion
Waterline corrosion Electrochemical machining animation
Electrochemical machining animation Action potential propagation
Action potential propagation Electrochemical series
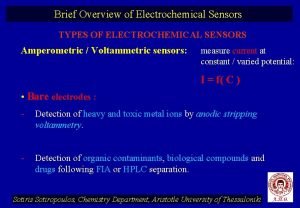
Electrochemical series Types of electrochemical sensors
Types of electrochemical sensors