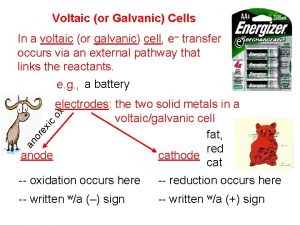
Electrochemistry Red Ox Part Deux Electrochemical cells Voltaic








- Slides: 17

Electrochemistry Red. Ox: Part Deux

Electrochemical cells Voltaic Converts chemical E from a Favorable Red. Ox reaction into electrical E Exothermic reaction a. k. a. battery Electrolytic Uses electrical E to force an UNFAVORABLE Red. Ox reaction to take place Endothermic reaction a. k. a. electroplating

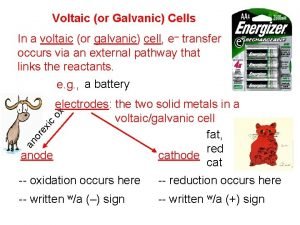
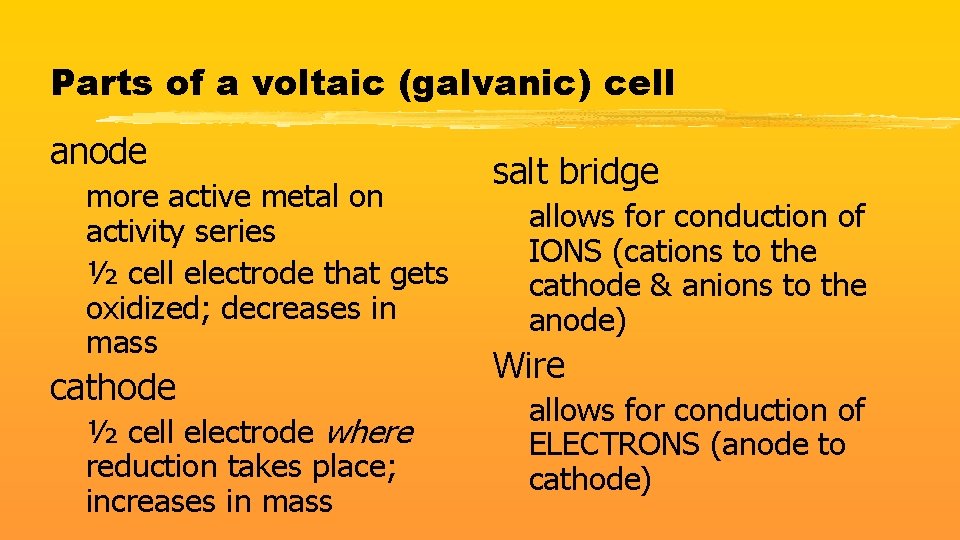
Parts of a voltaic (galvanic) cell anode more active metal on activity series ½ cell electrode that gets oxidized; decreases in mass cathode ½ cell electrode where reduction takes place; increases in mass salt bridge allows for conduction of IONS (cations to the cathode & anions to the anode) Wire allows for conduction of ELECTRONS (anode to cathode)

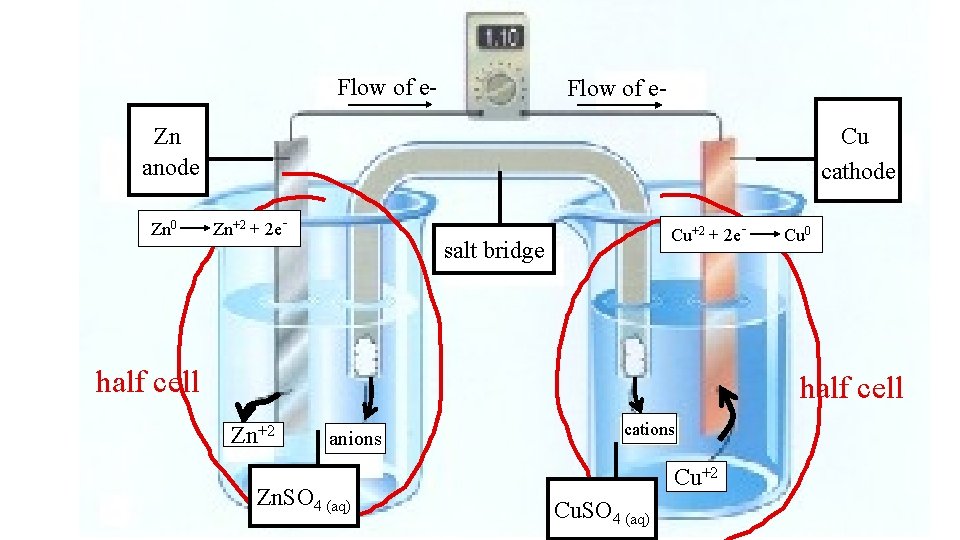
Flow of e- Flow of e. Cu cathode Zn anode Zn 0 Zn+2 + 2 e- Cu+2 + 2 e- salt bridge half cell Cu 0 half cell Zn+2 anions Zn. SO 4 (aq) cations Cu+2 Cu. SO 4 (aq)

Things to remember. . . e- flow Anode Cathode through wire more active metal less active metal Big Reduction Cathode Anode active Oxidation loses mass Ions flow across the bridge


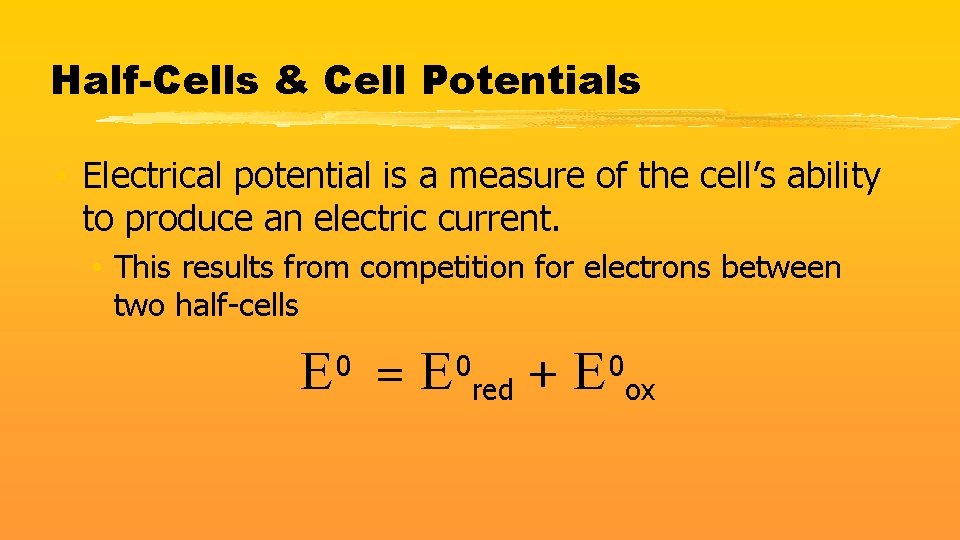
Half-Cells & Cell Potentials • Electrical potential is a measure of the cell’s ability to produce an electric current. • This results from competition for electrons between two half-cells E =E 0 0 red + E 0 ox

Standard Electrode Potentials • Standard Reduction potentials • The more positive the value, the easier the reduction half reaction takes place. • measured in volts

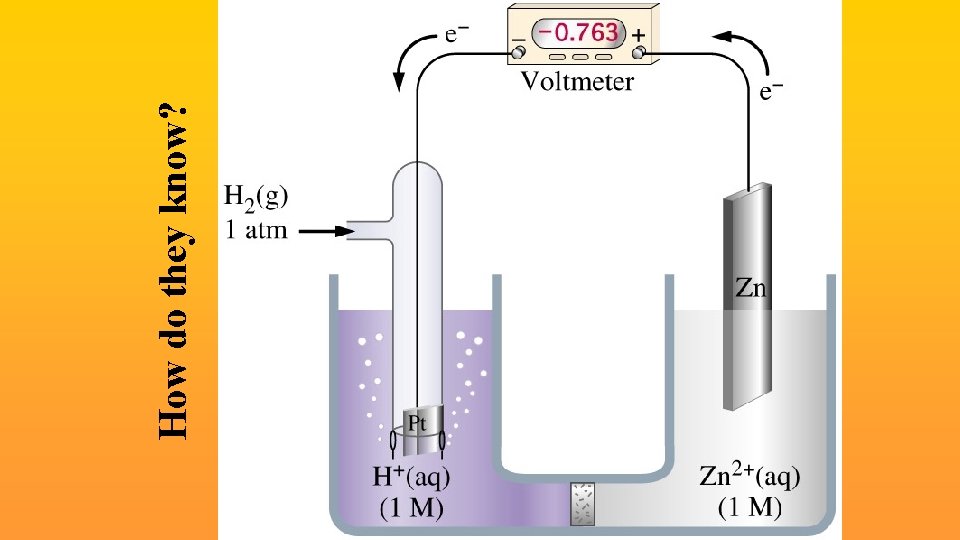
How do they know?

When using the formula. …. . switch the sign for oxidation & ADD If E 0 cell is positive, the reaction is favorable, Negative is UNfavorable Zero is at equilibrium (the battery is dead)

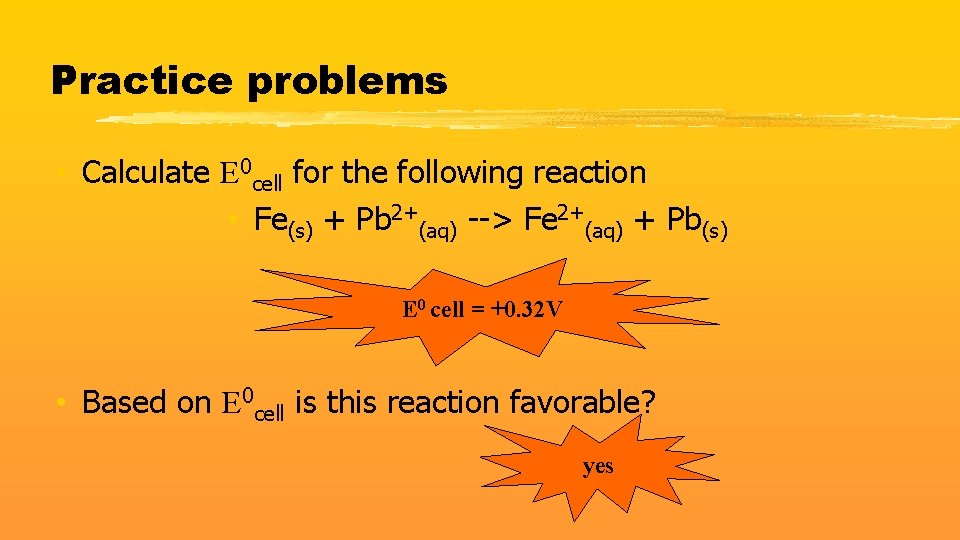
Practice problems • Calculate E 0 cell for the following reaction • Fe(s) + Pb 2+(aq) --> Fe 2+(aq) + Pb(s) E 0 cell = +0. 32 V • Based on E 0 cell is this reaction favorable? yes

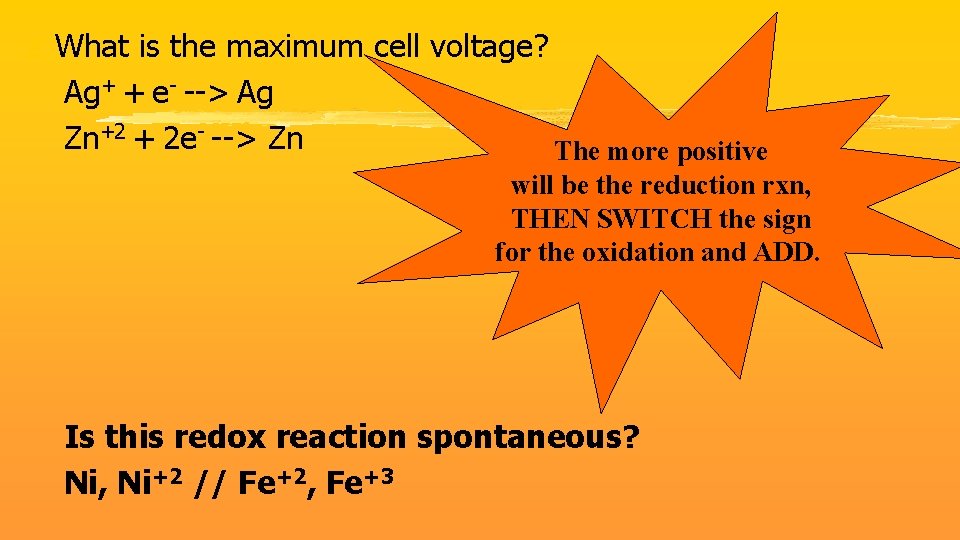
z What is the maximum cell voltage? Ag+ + e- --> Ag Zn+2 + 2 e- --> Zn The more positive will be the reduction rxn, THEN SWITCH the sign for the oxidation and ADD. Is this redox reaction spontaneous? Ni, Ni+2 // Fe+2, Fe+3

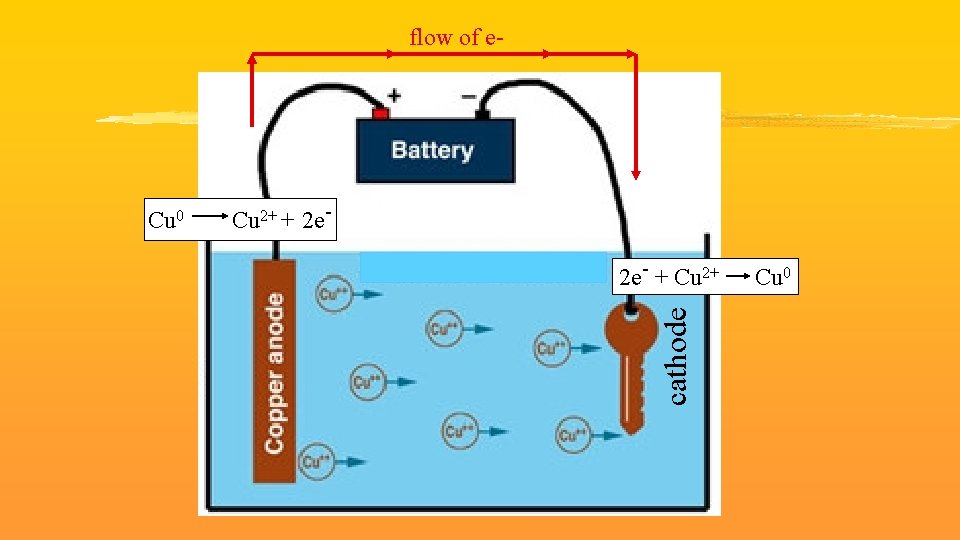
Electrolytic Cells UNfavorable Red. Ox reaction needs an outside power source to force e- to flow from anode to cathode Used for electroplating coating an inexpensive metal with an expensive metal a. k. a. Electrolysis

flow of e- Cu 2+ + 2 e 2 e- + Cu 2+ cathode Cu 0


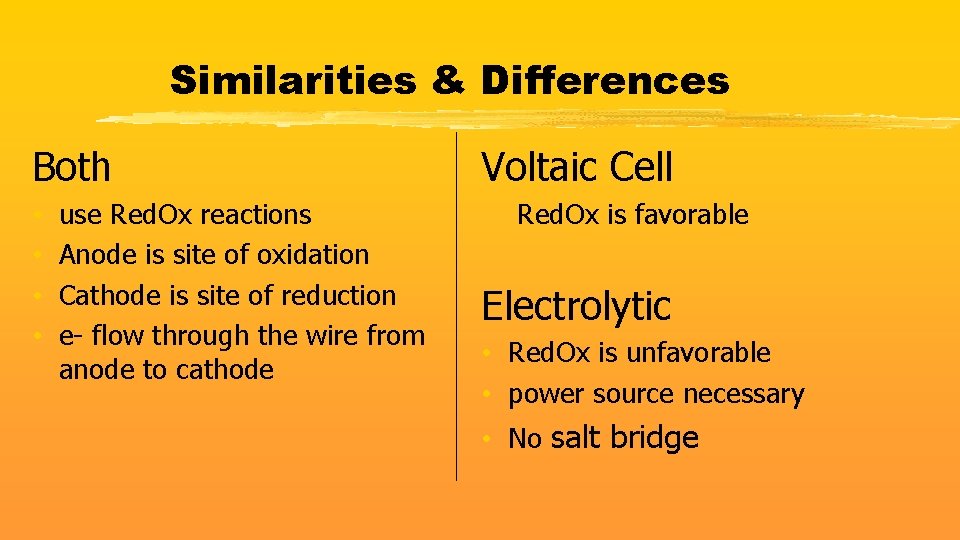
Similarities & Differences Both • • use Red. Ox reactions Anode is site of oxidation Cathode is site of reduction e- flow through the wire from anode to cathode Voltaic Cell Red. Ox is favorable Electrolytic • Red. Ox is unfavorable • power source necessary • No salt bridge

The End
 Voltaic cells example #2 worksheet answers
Voltaic cells example #2 worksheet answers Electrolysis vs voltaic cell
Electrolysis vs voltaic cell Voltaic and galvanic cells
Voltaic and galvanic cells Red blood cells and white blood cells difference
Red blood cells and white blood cells difference Voltaic aim
Voltaic aim Roger trinquier
Roger trinquier Balance redox
Balance redox Voltaic cell electron flow
Voltaic cell electron flow Lead acid battery primary or secondary
Lead acid battery primary or secondary Voltaic cell virtual lab
Voltaic cell virtual lab Voltaic fundamental
Voltaic fundamental Voltaic fundamentals
Voltaic fundamentals Voltaic fundamentals
Voltaic fundamentals Voltaic aim
Voltaic aim Paranasal sinus development
Paranasal sinus development Proximal convoluted tubule
Proximal convoluted tubule Thyroid parafollicular cells
Thyroid parafollicular cells How are mitosis and meiosis similar
How are mitosis and meiosis similar