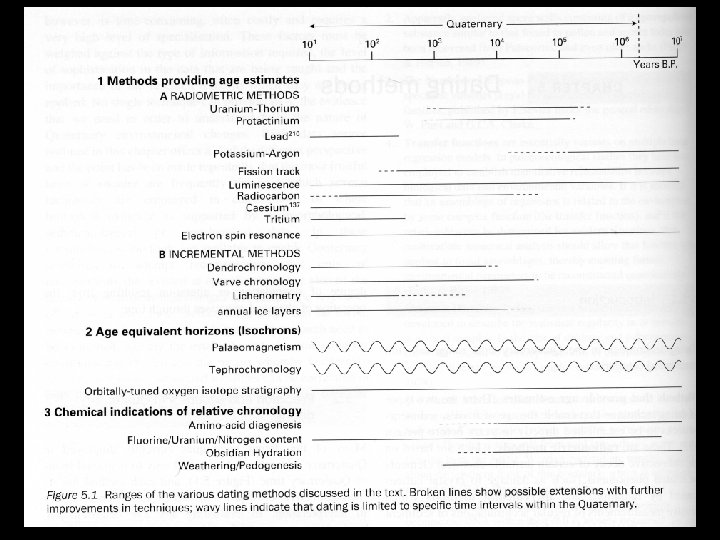
Quaternary Environments Dating Methods I Accuracy Versus Precision


























- Slides: 48

Quaternary Environments Dating Methods I


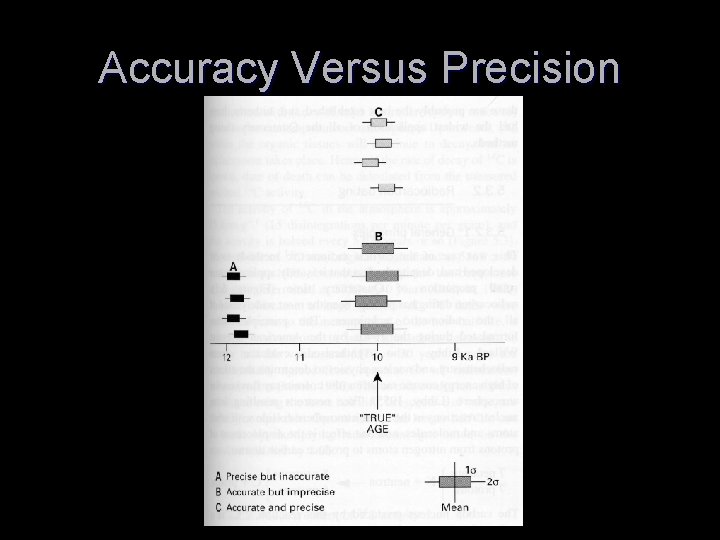
Accuracy Versus Precision T Precision means that the samples have low amount of error associated with the dating T Accuracy means that the samples are dated to the true age of the sample T We strive for both accuracy and precision in dating techniques

Accuracy Versus Precision

Relative Versus Absolute Dating T Relative Dating T Principle T Absolute of Superposition Dating T Provides solid chronological dates (within error bars) that are related to a calendar year scale

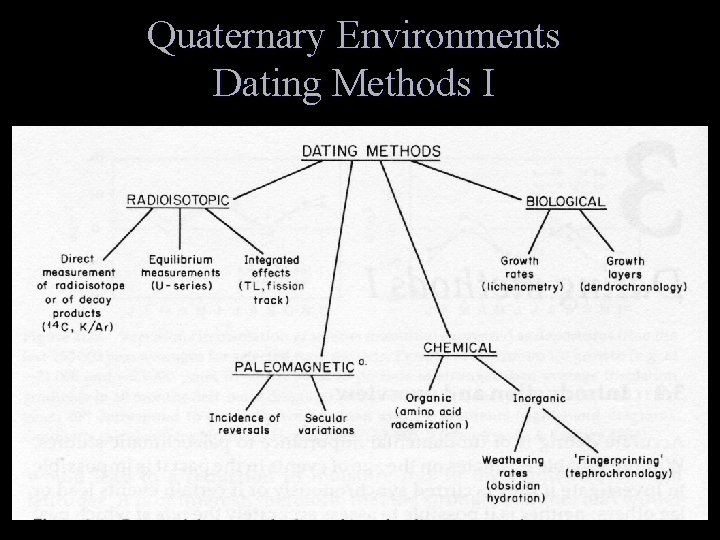
Methods T Radioisotopic Methods T T Paleomagnetic Methods T T Relies on past reversals of the Earth’s magnetic field Organic and Inorganic Chemical Methods T T Based on rate of atomic disintegration Based on time-dependent chemical changes in a sample Biological Methods T Based on the growth of an organism

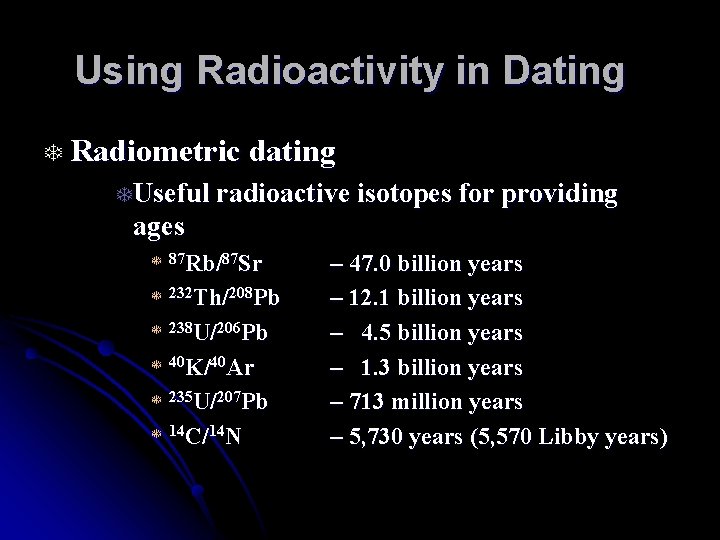
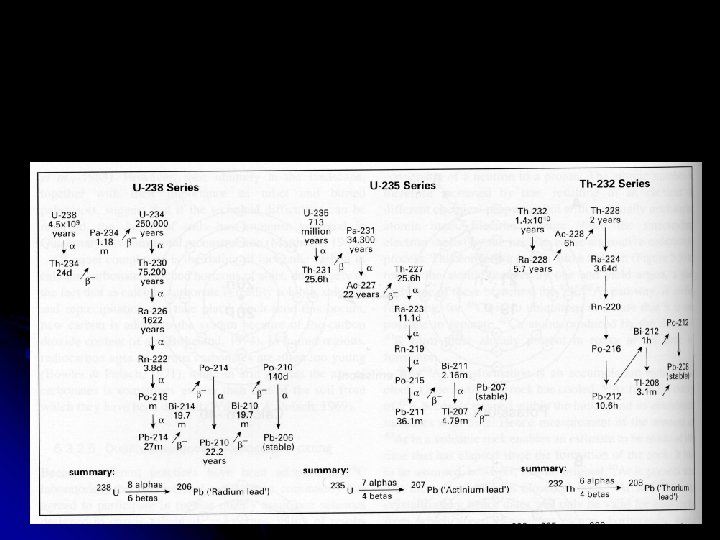
Using Radioactivity in Dating T Radiometric dating TUseful radioactive isotopes for providing ages T 87 Rb/87 Sr T 232 Th/208 Pb T 238 U/206 Pb T 40 K/40 Ar T 235 U/207 Pb T 14 C/14 N – 47. 0 billion years – 12. 1 billion years – 4. 5 billion years – 1. 3 billion years – 713 million years – 5, 730 years (5, 570 Libby years)

Sources of Error T T A closed system is required To avoid potential problems, only fresh, unweathered samples should be used

Using Radioactivity in Dating T Reviewing basic atomic structure TNucleus T Protons – positively charged particles with mass T Neutrons – neutral particles with mass T Electrons – negatively charged particles that orbit the nucleus

Using Radioactivity in Dating T Reviewing basic atomic structure TAtomic number T An element’s identifying number T Equal to the number of protons in the atom’s nucleus TMass number T Sum of the number of protons and neutrons in an atom’s nucleus T Identifies an isotope

Using Radioactivity in Dating T Reviewing basic atomic structure TIsotope T Variant of the same parent atom T Differs in the number of neutrons T Results in a different mass number than the parent atom

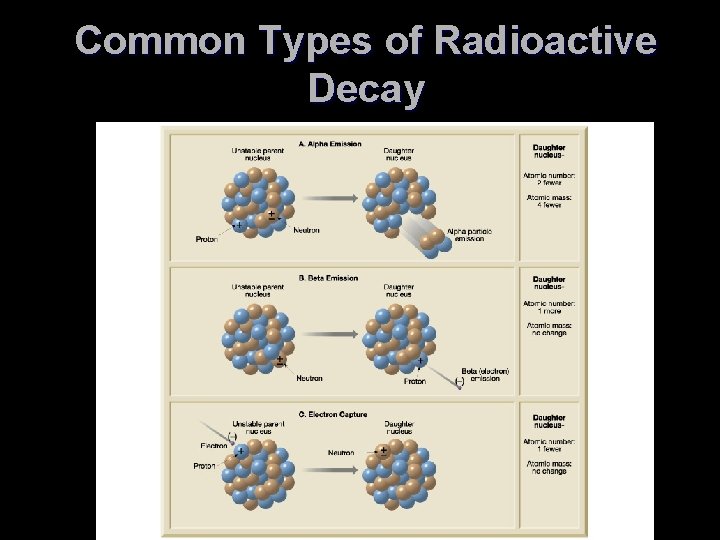
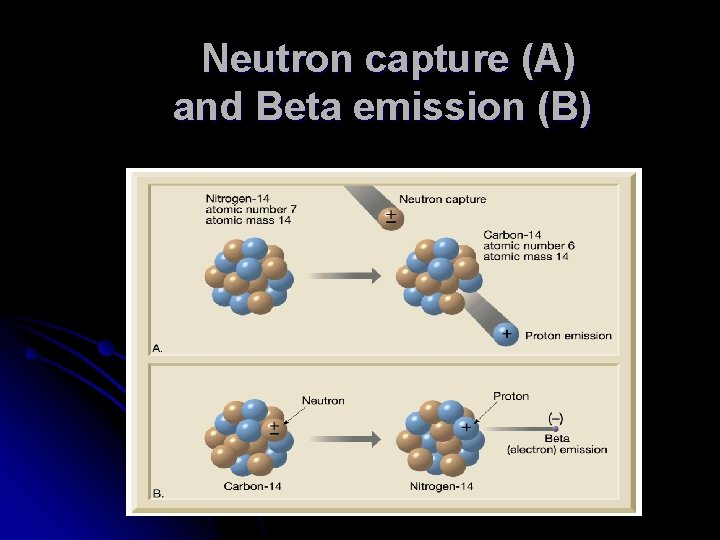
Using Radioactivity in Dating T Radioactivity TSpontaneous changes (decay) in the structure of atomic nuclei T Types of radioactive decay TAlpha emission T Emission of 2 protons and 2 neutrons (an alpha particle) T Mass number is reduced by 4 and the atomic number is lowered by 2

Using Radioactivity in Dating T Types of radioactive decay TBeta emission T An electron (beta particle) is ejected from the nucleus T Mass number remains unchanged and the atomic number increases by 1

Using Radioactivity in Dating T Types of radioactive decay TElectron capture T An electron is captured by the nucleus T The electron combines with a proton to form a neutron T Mass number remains unchanged and the atomic number decreases by 1

Common Types of Radioactive Decay

Using Radioactivity in Dating T Parent – an unstable radioactive isotope T Daughter product – the isotopes resulting from the decay of a parent T Half-life – the time required for one-half of the radioactive nuclei in a sample to decay

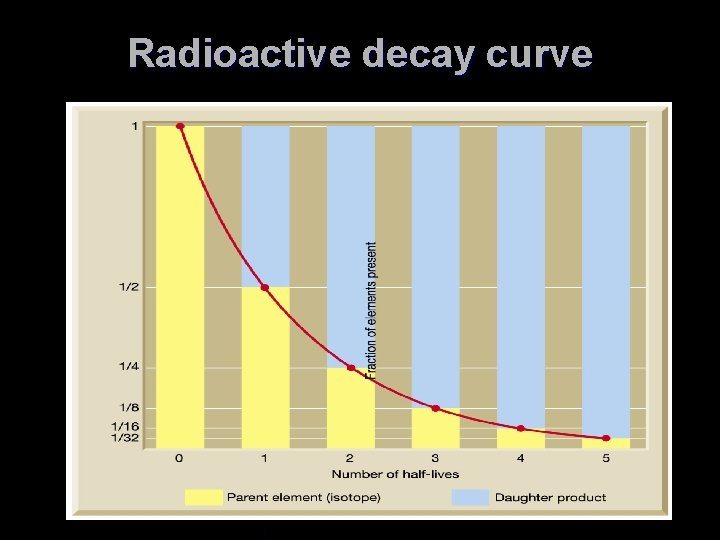
Using Radioactivity in Dating T Radiometric dating TPrinciple of radioactive dating T The percentage of radioactive atoms that decay during one half-life is always the same (50 percent) T However, the actual number of atoms that decay continually decreases T Comparing the ratio of parent to daughter yields the age of the sample

Radioactive decay curve

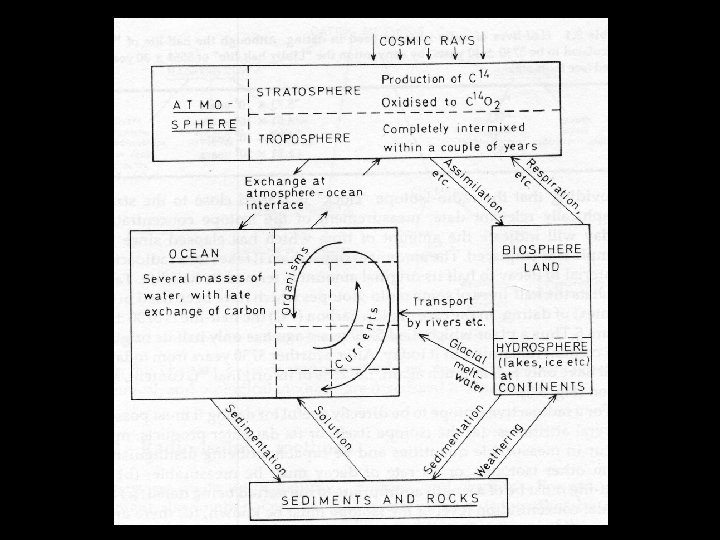
Radiocarbon Dating T Dating with 14 C THalf-life of 5730 years TUsed to date very recent events T 14 C is produced in the upper atmosphere TUseful tool for anthropologists, archeologists, and geologists who study very recent Earth history


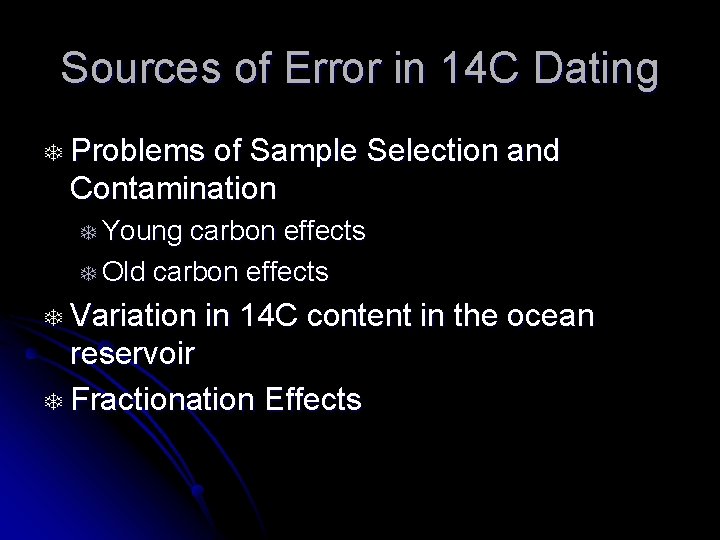
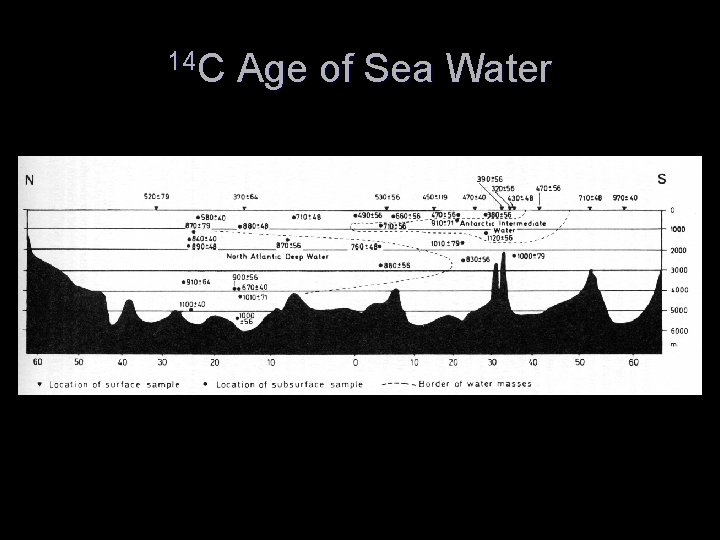
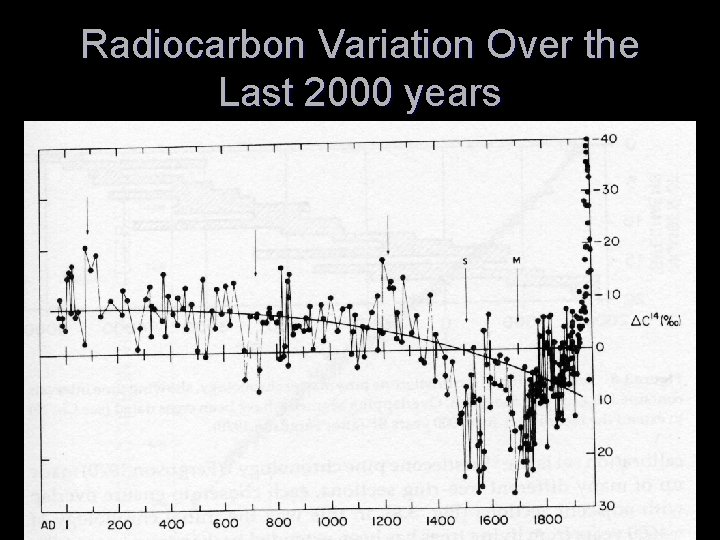
Sources of Error in 14 C Dating T Problems of Sample Selection and Contamination T Young carbon effects T Old carbon effects T Variation in 14 C content in the ocean reservoir T Fractionation Effects

14 C Age of Sea Water

Radiocarbon Variation Over the Last 2000 years

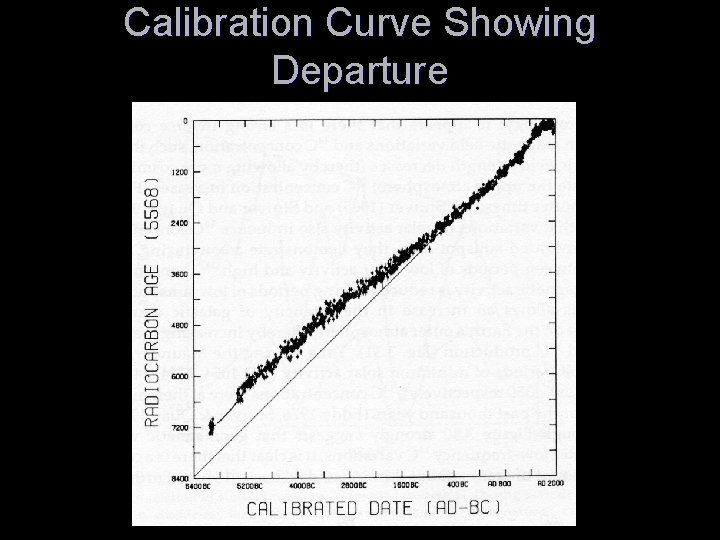
Calibration Curve Showing Departure

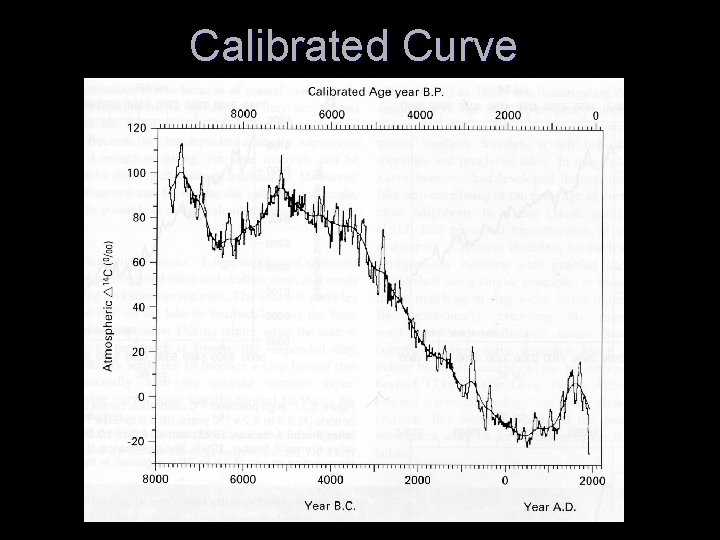
Calibrated Curve

Radiocarbon Plateaus

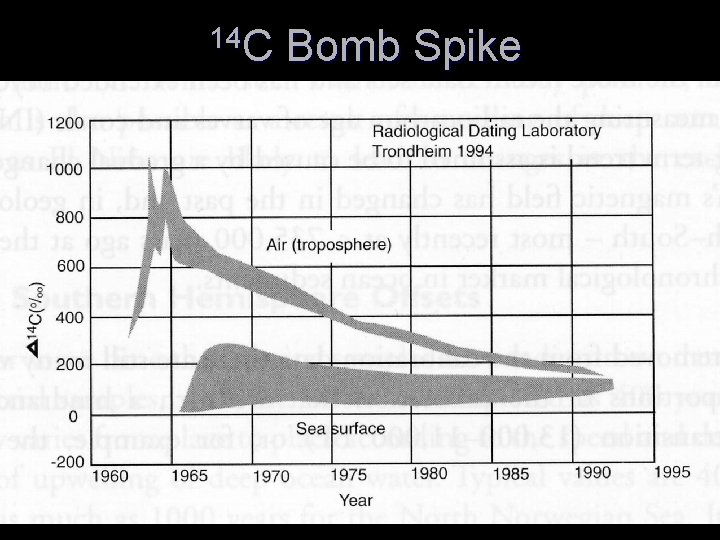
14 C Bomb Spike

Potassium Argon Dating (40 K/40 Ar) T Instrumental in dating sea-floor basalts and providing the timing of magnetic reversals T Used in dating lava flows T Also for dating metamorphic events

Potassium Argon Dating (40 K/40 Ar) and 41 K, Stable T 40 K unstable and 0. 012% of all potassium T 40 K decays to 40 Ca and 40 Ar T Ca is common in rocks and is therefore not useful in dating T Measure the amount of 40 Ar in the lab and the amount of 40 K is also measured from the sample T 39 K

Potassium Argon Dating (40 K/40 Ar) T T T Long half-life makes this useful over long time scales but not really usable at less than 100, 000 years Optimal time range is around 30 ma and up to 1 ba rocks can be dated Dating is done on sanidine, plagioclase, biotite, hornblende, and olivine in volcanic rocks and glauconite, feldspar, and sylvite in sedimentary rocks

Problems of 40 K/40 Ar T Assumptions No Ar was left in the rock at formation T System has remained closed since formation T T Checks T T The ratio of 36 Ar to 40 Ar is known in the atmosphere and can be measured in the rock to determine atmospheric contamination Problem T Loss of Ar due to diffusion, recrystallization, solution, and chemical reactions

40 Ar/39 Ar T T T Dating A problem with 40 K/40 Ar dating is that K and Ar are measured at different places in the rock This can be solved by irradiating the samples and converting 39 K to 39 Ar With the known ratio of 40 K to 39 K, the amount of 40 K can be calculated from the same lattice structure as the 39 Ar

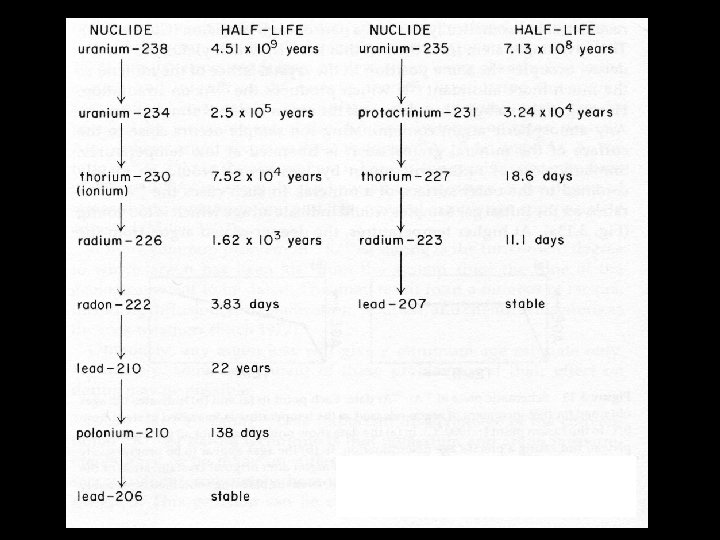
Uranium Series Dating T T T 238 U and 235 U have a decay process that cascades through a series of elements Each decay stage can be used as a dating tool Thermal Ionization Mass Spectrometry (TIMS) allows very accurate estimates from small samples U series are useful in dating corals and speleothems Mollusks seem to be an open system in relation to U and are not generally conducive to U series dating


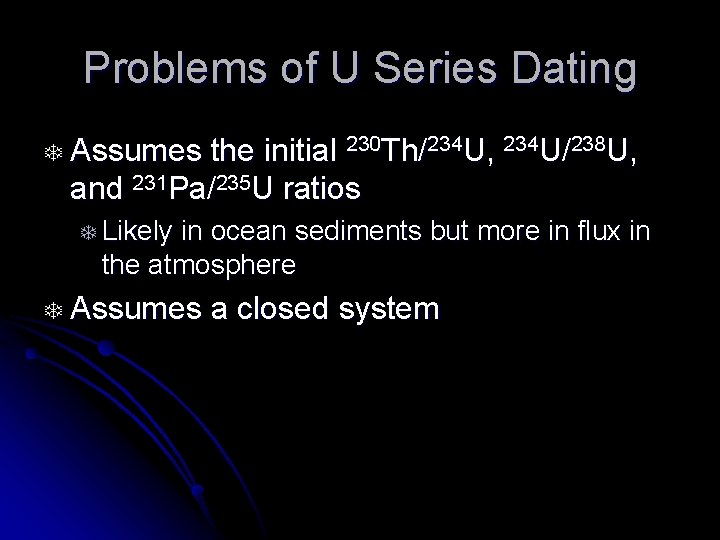
Problems of U Series Dating T Assumes the initial 230 Th/234 U, 234 U/238 U, and 231 Pa/235 U ratios T Likely in ocean sediments but more in flux in the atmosphere T Assumes a closed system

Luminescence Dating Principles and Applications T T T Light emitted from a mineral crystal (usually quartz or feldspars) when exposed to heat or light The light emitted is related to the amount of ionizing radiation that the sample has been exposed to from sediment The clock is set to zero by heating or optical bleaching T Therefore Loess and fluvial sediments make good candidates for luminescence dating

Luminescence T Thermoluminescence T When T (TL Dating) the light is emitted as a result of thermal hearting T First published Wintle and Huntley 1979 Optical and Infrared Stimulated Luminescence (OSL and IRSL Dating) T Visible or infrared energy emitted in response to radiation

Problems in TL Dating T T T Assumes that the relationship between the radiation dose and the resulting luminescence is a linear relationship; not always the case T <5, 000 yrs the rate of electron accumulation is slow, possibly needing to exceed a threshold T Some minerals may reach saturation >300, 000 yrs Anomalous Fading – Minerals do not hold the electrons beyond a few weeks Variations in environmental dose; related to moisture content for one example

Optical and Infrared Stimulated Luminescence (OSL and IRSL Dating) T T T Zero in the modern sediments Sensitive to light bleaching setting the system to zero Multiple measurements are possible because short stimulation to the light source does not deplete the potential luminescence


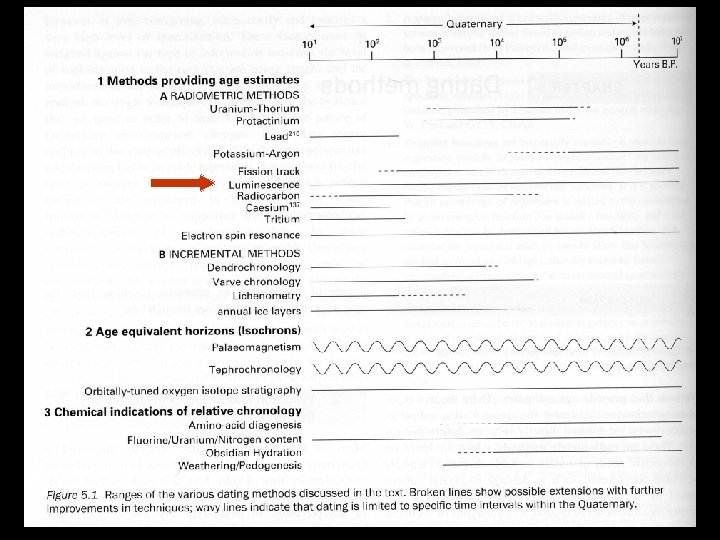
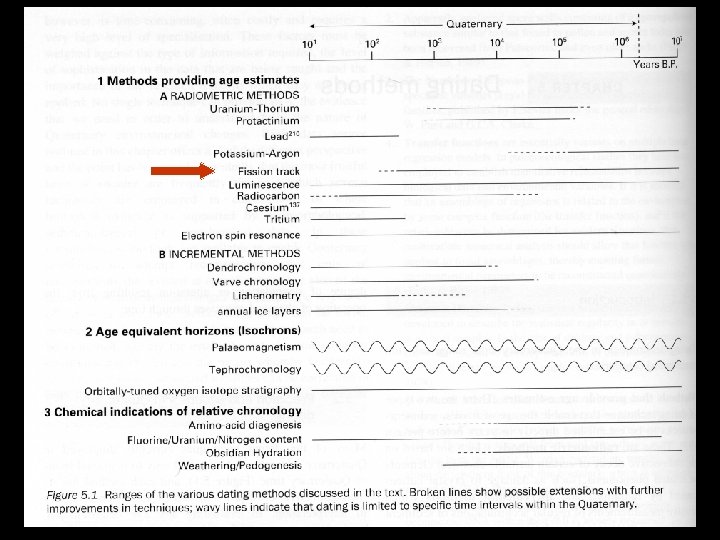
Fission Track Dating T T Uranium will decay through fission, splitting the nucleus and shooting the two halves into the mineral The results are fission tracks from 10 -20μm in length Some glassy minerals will loose their fission tracks through heating, setting the clock to zero Different minerals have different annealing temperatures

Fission Track Dating T T The samples are polished and etched with a chemical that brings out the tracks The tracks are counted, then the sample is heated, annealing the tracks. Then the sample is irradiated with a slow neutron beam and the tracks from the fission of 235 U are counted The number of induced tracks is proportional to the amount of 238 U in the sample The known fission rate of 238 U is used to calculate the age of the sample

Fission Track Dating T T T Can be used in apatite, micas, sphene, and zircons Can also be used in rocks such as volcanic ash, obsidian, basalts, granites, tuffs, and carbonatites Ranges from 103 to 108 years The error associated with this technique is hard to determine and is seldom reported Repeat measures are the best, but are seldom undertaken because of time constraints




Dendrochronology T Temperate trees produce annual rings. T The trees are recording all of the environmental variables that affect tree growth. T Can be used to date objects with annual resolution back 10, 000 years in the best circumstances.

Neutron capture (A) and Beta emission (B)