Quantum Physics The Fundamental Forces Strength of forces






















- Slides: 44

Quantum Physics

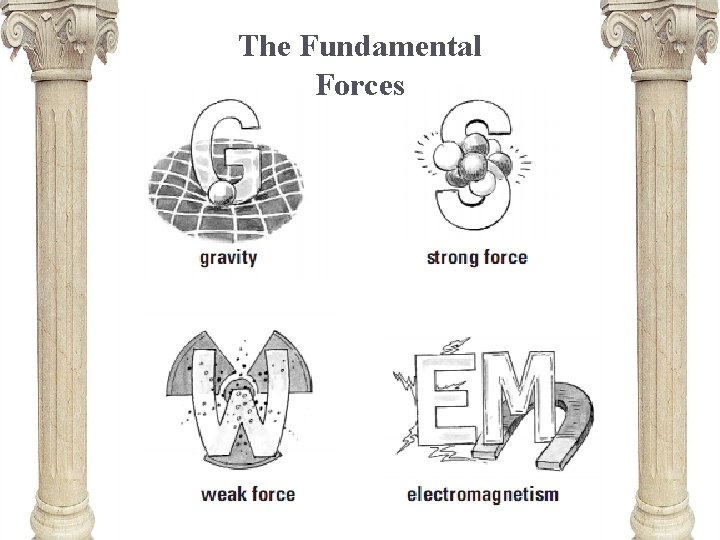
The Fundamental Forces

Strength of forces • Although gravity acts over long distances, it is the weakest! • The strongest is…the strong force. • Followed by E&M, the weak, and finally gravity.

Forces Gravitational Electromagnetic • Weak • Attraction between objects • Strong • Often used to hold chemical bonds together (attraction of protons and electrons)

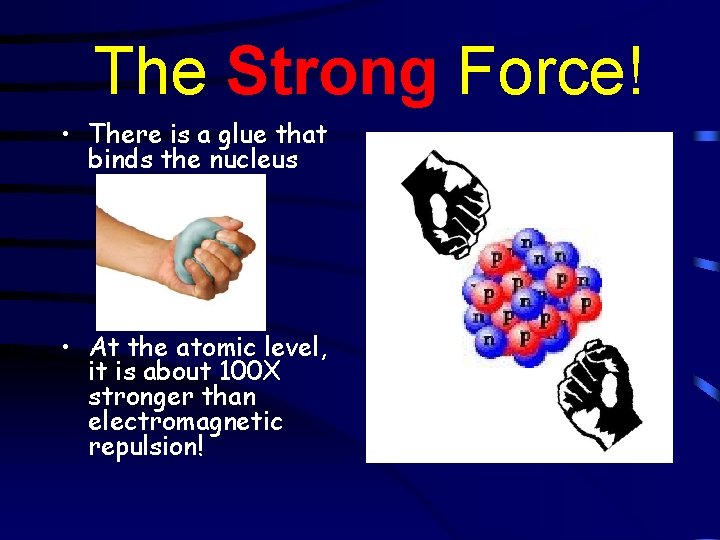
The Strong Force! • There is a glue that binds the nucleus • At the atomic level, it is about 100 X stronger than electromagnetic repulsion!

The Weak Force • Radioactive decay, namely beta decay • Beta decay is the emission of electrons when a neutron is changed into a proton and an electron • Beta decay is also responsible for initiating hydrogen fusion in stars

Wave-Particle Duality • Light can behave like a wave or like a particle • Wave = transverse (EM wave) • Particle = photons

It is easy to imagine light behaving as either. This debate went on for centuries!

A sand dune is made of: Continuous waves & Discrete, individual sand grains We use similar terms for light! Waves: Continuous light Photons: Discrete light

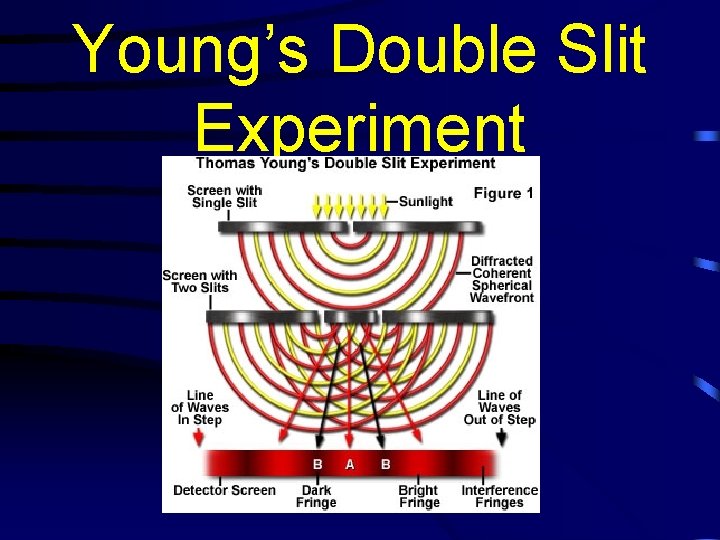
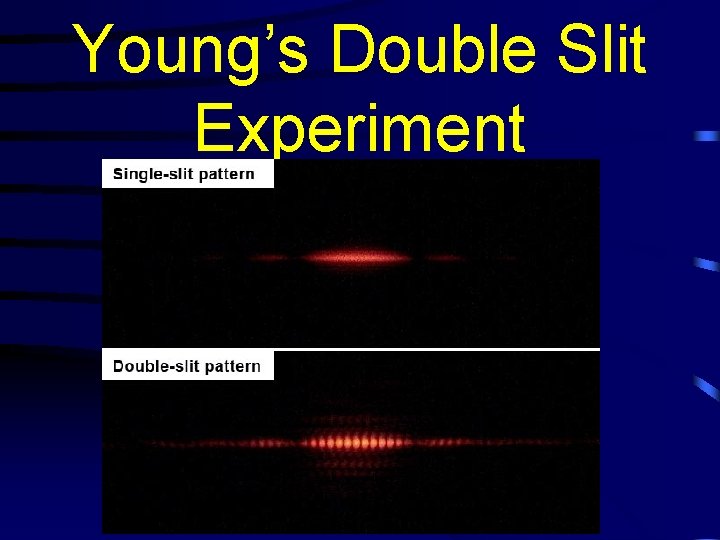
Young’s Double Slit Experiment

Young’s Double Slit Experiment

Young’s Double Slit Experiment

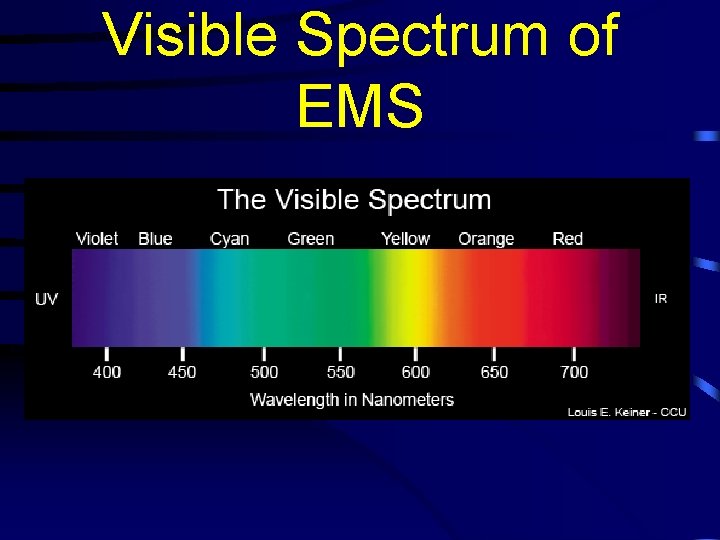
Reminders • Visible light – part of the EMS ranging between 400 nm to 700 nm • Ultraviolet light – Electromagnetic waves with wavelengths below 400 nm • Infrared light – Electromagnetic waves with wavelengths above 700 nm

Visible Spectrum of EMS

Reminders • The colors of the spectrum are: • Red, orange, yellow, green, blue, indigo, violet • Lowest energy = red • Highest energy = violet

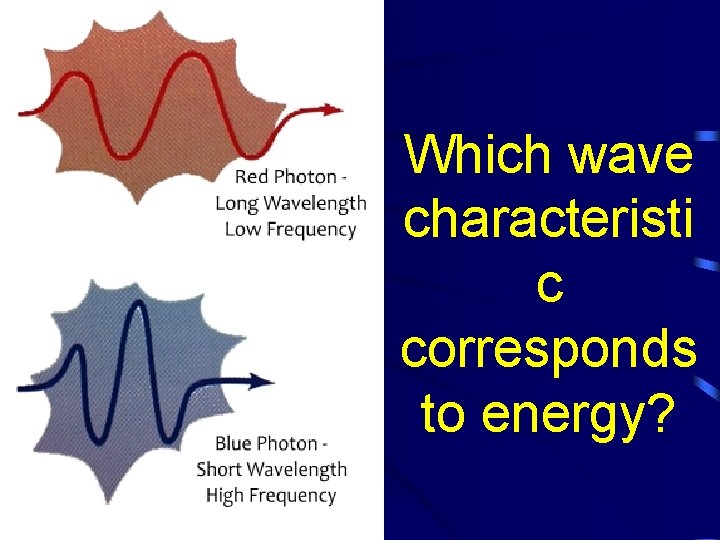
Which wave characteristi c corresponds to energy?

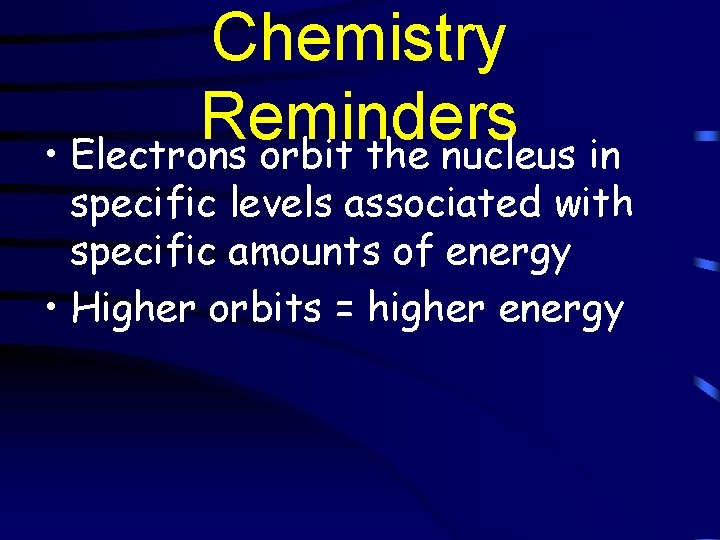
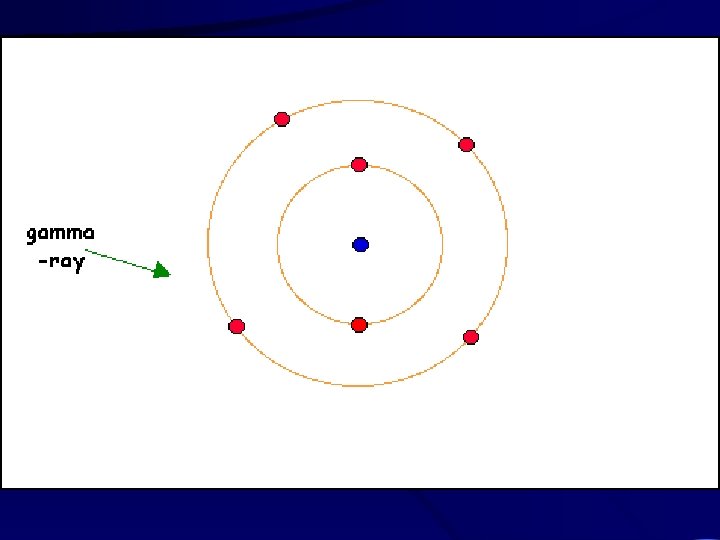
Chemistry Reminders • Electrons orbit the nucleus in specific levels associated with specific amounts of energy • Higher orbits = higher energy

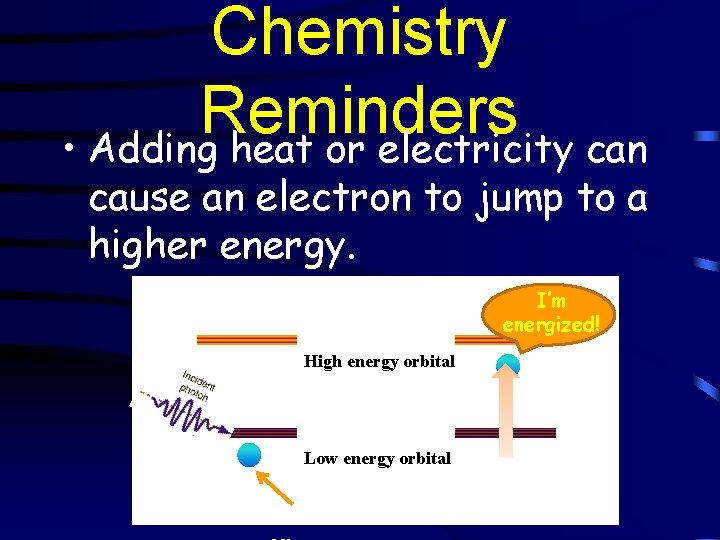
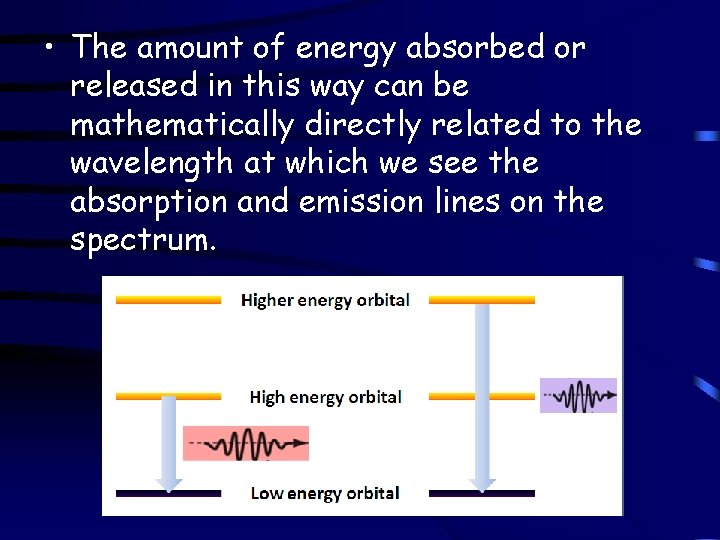
Chemistry Reminders • Adding heat or electricity can cause an electron to jump to a higher energy. I’m energized! High energy orbital Low energy orbital Electr

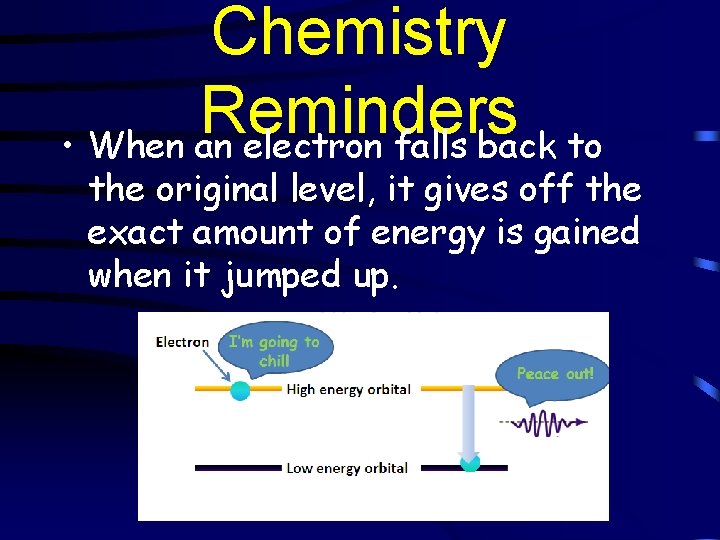
Chemistry Reminders • When an electron falls back to the original level, it gives off the exact amount of energy is gained when it jumped up.

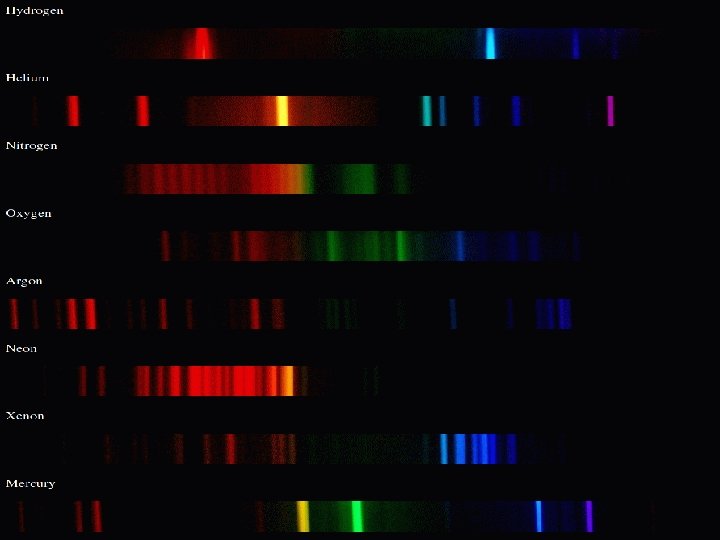
Spectroscopy • Pertains to the dispersion of light into its available spectrum • Two types: – Continuous – Discrete

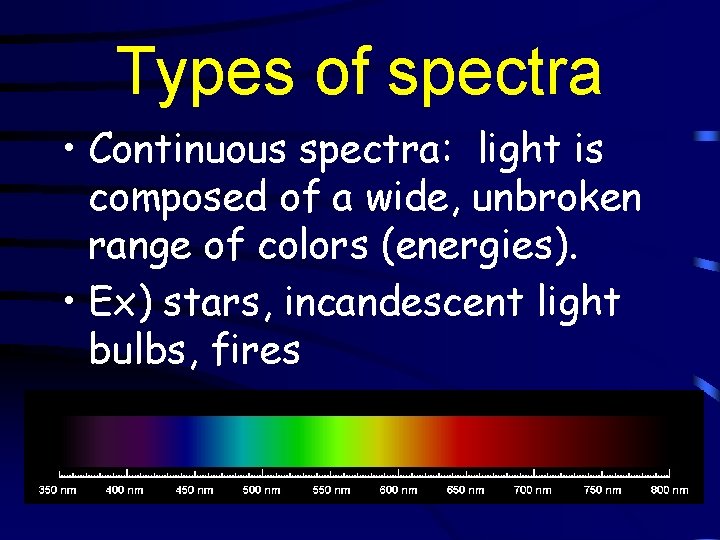
Types of spectra • Continuous spectra: light is composed of a wide, unbroken range of colors (energies). • Ex) stars, incandescent light bulbs, fires

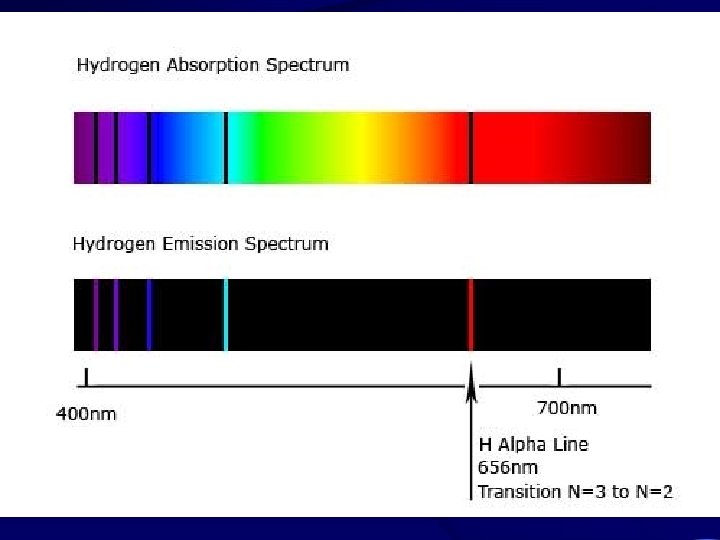
Types of Spectra • Discrete spectra: only bright or dark lines at very distinct and sharply -defined colors (energies).

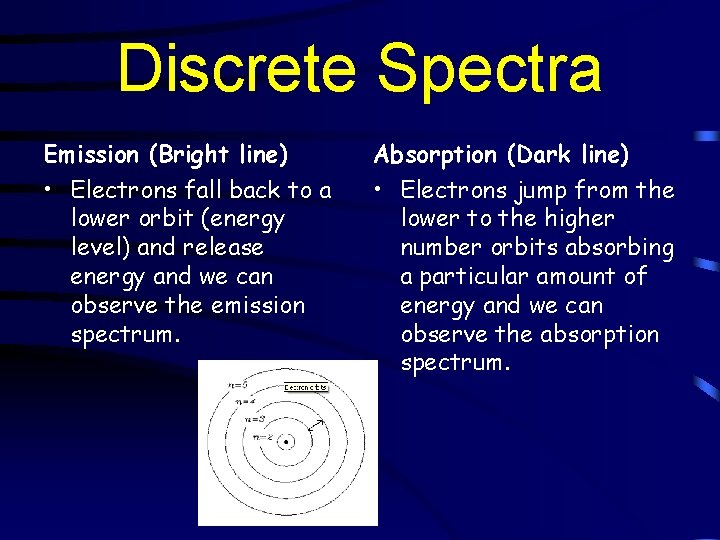
Discrete Spectra Emission (Bright line) Absorption (Dark line) • Electrons fall back to a lower orbit (energy level) and release energy and we can observe the emission spectrum. • Electrons jump from the lower to the higher number orbits absorbing a particular amount of energy and we can observe the absorption spectrum.

• The amount of energy absorbed or released in this way can be mathematically directly related to the wavelength at which we see the absorption and emission lines on the spectrum.


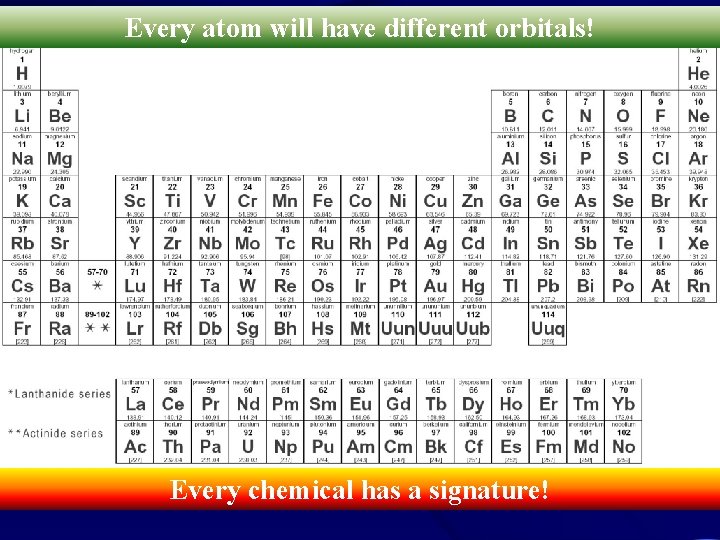
Every atom will have different orbitals! Every chemical has a signature!


This is how you get different colored fireworks and “neon” signs!

Key Ideas • Quantized – having a specific, discrete amount • Photon – a discrete bundle of energy which has no mass

Photoelectric Effect - What happens? • A photon of light hits an object. • Energy is transferred from the photon to the object. • If the energy is great enough to break a bond, an electron will be thrown off.


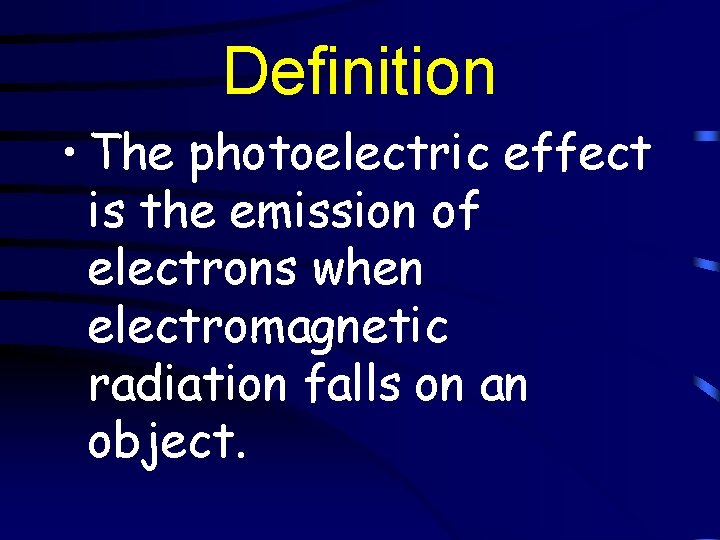
Definition • The photoelectric effect is the emission of electrons when electromagnetic radiation falls on an object.

Threshold frequency • The minimum amount of energy required to eject an electron. • If the energy is below the threshold frequency, there is no emission • If the energy is equal to or greater than the threshold frequency, an electron will be emitted

Threshold frequency • Different substances have different threshold frequencies. • Example: all wavelengths of visible light except red will eject electrons from cesium. However, UV light is required for zinc.

Click for simulation

So What Happened? • As photon (light) energy increased, what happened to the current created by the free electrons? • It increased!

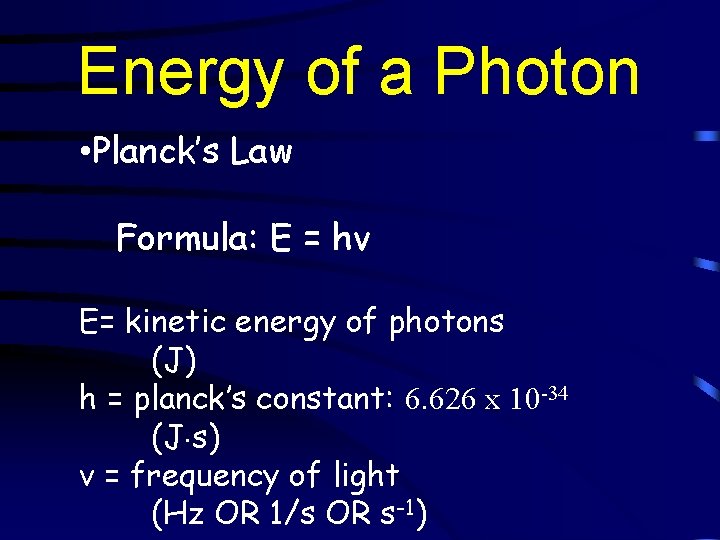
Energy of a Photon • Planck’s Law Formula: E = hv E= kinetic energy of photons (J) h = planck’s constant: 6. 626 x 10 -34 (J s) v = frequency of light (Hz OR 1/s OR s-1)

Energy of a Photon • Planck’s Law • Used for calculating the energy of a photon • Ex: If you know the frequency/color of light that starts causing the photoelectric effect, you can easily calculate the actual energy needed to knock that electron out of the atom.

Practice Orange light is the threshold frequency of Cesium. Orange’s frequency is: 4. 00 x 1014 Hz…how many Joules of energy was required to eject the electron from the atom of Cesium?

Practice Orange light is the threshold frequency of Cesium. Orange’s frequency is: 4. 00 x 1014 Hz…how many Joules of energy was required to eject the electron from the atom of Cesium? • E = hv • E = (6. 626 x 10 -34) x (4. 00 x 1014)

Scientists • Max Planck – discovered the quantized nature of energy

Scientists • Albert Einstein – Proposed that light can behave as a wave or a particle which he called photons • Elaborated on photoelectric effect to include threshold frequency

Scientists • Niels Bohr – created quantized model of an atom

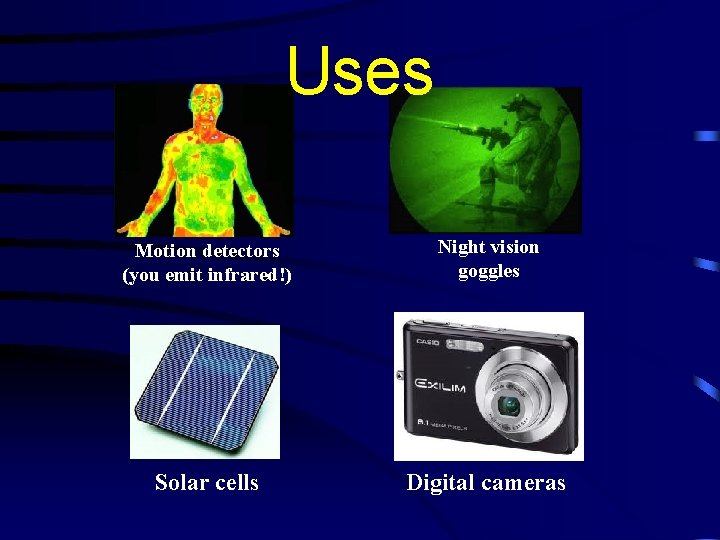
Uses Motion detectors (you emit infrared!) Night vision goggles Solar cells Digital cameras