Percentage Ratio Strength and Other Expressions of Concentration
































- Slides: 32

Percentage, Ratio Strength, and Other Expressions of Concentration Yewande Dayo Student Pharmacist

Objectives � Define percent weight-in-volume, percent volume-involume, and percent weight-in-weight. � Define ratio strength. � Convert percent strength to ratio strength and ratio strength to percent strength. � Calculate the percent strength and ratio strength of a pharmaceutical preparation. � Apply percent strength and ratio strength to calculate the quantity of an ingredient present in a pharmaceutical preparation. � Apply percent strength and ratio strength to calculate the quantity of an ingredient to use in compounding a pharmaceutical preparation.

Percentage Strength � Percentage strength and its corresponding sign (%) means rate per hundred � 50% = 50 parts in 100 = 0. 50 � The pharmacist usually encounters liquid preparations where the parts represent grams of ingredient in 100 m. L of liquid preparation.

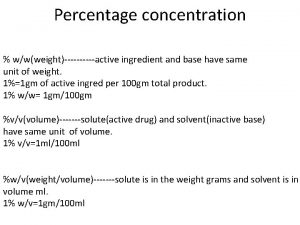
Percentage Preparations � Percent Weight in Volume (w/v): number of grams in 100 m. L of solution. Expressed as: ______%w/v � Percent Volume in Volume (v/v): number of milliliters in 100 m. L of solution. Expressed as: ______%v/v � Percent weight in weight (w/w): number of grams in 100 grams of solution. Expressed as: ______%w/w

Assumptions � If the term % is used without qualification the following are assumed: � mixtures of solids and semisolids (%w/w) � solutions or suspensions of solid in liquid (%w/v) � solutions of liquid in liquid (%v/v)

Example 1 � How many milligrams of ephedrine sulfate should be used in preparing the following prescription? Rx Sol. Ephedrine Sulfate ½% Sig. For the nose 30 m. L

Example 2 � How many milligrams of certified red color should be used in preparing 5 liters of a 0. 01% solution?

Example 3 � If a pharmacist dissolves the contents of eight capsules containing 250 mg clindamycin HCl per capsule into sufficient amount of astringent liquid to prepare 120 m. L of solution, what is the percentage strength (w/v) of clindamycin HCl in the prescription?

Example 4 � If 425 g of sucrose is dissolved in enough water to make 500 m. L, what is the percentage strength of the solution?

Example 5 �A sunscreen lotion contains 5% (v/v) of methyl salicylate. How many milliliters of methyl salicylate should be used in preparing 5 pints of the lotion?

Example 6 � What is the percentage strength (v/v) if 225 g of a liquid having a specific gravity of 0. 8 are added to enough water to make 1. 5 liters of the solution?

Example 7 � How many grams of a drug substance should be dissolved in 1800 m. L of water to make a 10% (w/w) solution?

Example 8 � How many grams of potassium permanganate should be used in compounding the following prescription � Rx � Potassium Permanganate 0. 02% � Purified water ad 250 m. L

Example 9 � If 500 g of dextrose are dissolved in 600 m. L of water with a resultant final volume of 1 liter, what is the percentage strength of dextrose in the solution on a w/w basis?

Example 10 � One pound of fungicidal ointment contains 9. 08 g of undecylenic acid. Calculate the percentage w/w of undecylenic acid in the ointment.

Final volume in w/w � It is usually impossible to prepare a final specific volume of % w/w solution because the volume displaced by the active drug cannot be known in advance.

Example 11 � How would you prepare 100 m. L of a 2% (w/w) solution of a drug substance in a solvent having a specific gravity of 1. 25?

Example 12 � If 1500 m. L of a solvent with a specific gravity 0. 85 are used to dissolve 75 g of a drug substance, what is the percentage strength (w/w) of the solution?

Example 13 � If 5 g of boric acid is dissolved in 100 m. L of water, what is the percentage strength (w/w) of the solution?

Example 14 � If 1000 m. L of syrup with a specific gravity of 1. 313 contains 850 g of sucrose, what is its percentage strength (w/w)?

Example 15 � What weight of a 5% (w/w) solution can be prepared from 2 g of active ingredient?

Ratio Strength � Solids in liquids = 1: 1000 = 1 g in 1000 m. L of solution � Liquids in liquids = 1: 1000 = 1 m. L in 1000 m. L of solution. � Solids in solids = 1: 1000 = 1 g in 1000 g of mixture.

Example 16 � Express 0. 02% as a ratio strength and 1: 4000 as percentage strength.

Example 17 � How many mg of gentian violet should be used in preparing the following solution? Rx Gentian violet solution 500 m. L 1: 10, 000 Sig. Instill as directed

Example 18 � How many mg of hexachlorophene should be used in compounding the following prescription? Rx Hexachlorophene 1: 400 Hydrophilic Oint. ad 10 g Sig. Apply.

Conversion to mg/m. L � Convert 4% (w/v) to mg/m. L

Example 19 � Convert 1: 10, 000 (w/v) to mg/m. L � Convert a product concentration of 1 g per 250 m. L to mg/m. L

Parts per Million (PPM) � 1 part of solute per 1 million part of the solution, e. g. , 1: 1, 000

Example 20 � Express 5 ppm of iron in water in percent strength and ratio strength.

Problem 21 Rx Gentamicin (40 mg/m. L) Inj. Gentamicin (0. 3%) Oph. Soln. 2 m. L 5 m. L Calculate the percent concentration of gentamicin in this fortified ophthalmic solution.

Problem 22 � Mellaril-S Suspension contains 25 mg of thioridazine per 5 m. L of suspension. What is the percentage strength (w/v) of thioridazine in the suspension?

� Rx � Ofloxacin Opthalmic Solution 0. 3% � Disp 10 m. L � Sig: 2 drops into eyes q 4 hours x 2 days; then 2 drops q 6 hours x 4 days � How many grams of Ofloxacin should be used in preparing the prescription
 Percentage strength
Percentage strength What is ratio strength
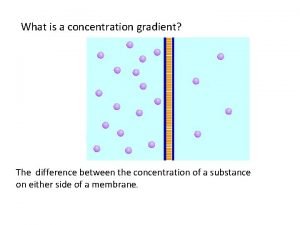
What is ratio strength Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Strength vs concentration
Strength vs concentration Percentage concentration
Percentage concentration Yield and tensile strength
Yield and tensile strength Darrow solution half strength
Darrow solution half strength Altering product strength
Altering product strength Difference between ratio and percentage
Difference between ratio and percentage Four firm concentration ratio
Four firm concentration ratio Concentration ratio
Concentration ratio Four firm concentration ratio
Four firm concentration ratio Four-firm concentration ratio
Four-firm concentration ratio 4 firm concentration ratio
4 firm concentration ratio The four-firm concentration ratio
The four-firm concentration ratio The four-firm concentration ratio
The four-firm concentration ratio Ratio to percentage
Ratio to percentage Pharmacy dilution calculations pdf
Pharmacy dilution calculations pdf High strength to weight ratio
High strength to weight ratio Glider horizontal stabilizer
Glider horizontal stabilizer Best strength to weight ratio bridge design
Best strength to weight ratio bridge design Acid test ratio and quick ratio
Acid test ratio and quick ratio Genotypic ratio and phenotypic ratio
Genotypic ratio and phenotypic ratio Current ratio and quick ratio
Current ratio and quick ratio Types of position
Types of position Rr statistics
Rr statistics Velocity ratio in gear
Velocity ratio in gear Primary reinforcer
Primary reinforcer Risiko relatif dan odds ratio
Risiko relatif dan odds ratio Base and rate and percentage
Base and rate and percentage Answer
Answer Concentration = moles / volume
Concentration = moles / volume