THE MOLE A guide for A level students















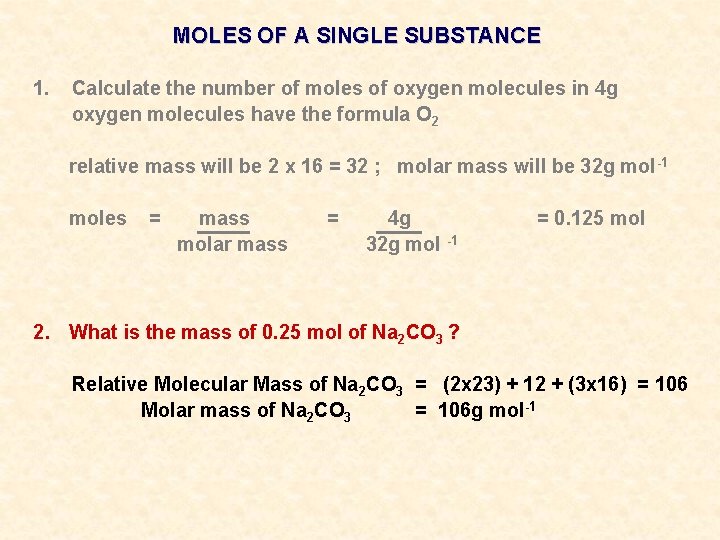
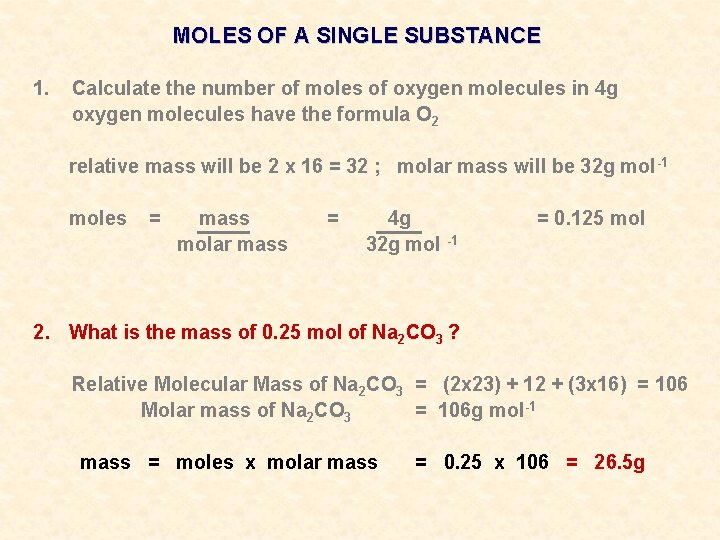
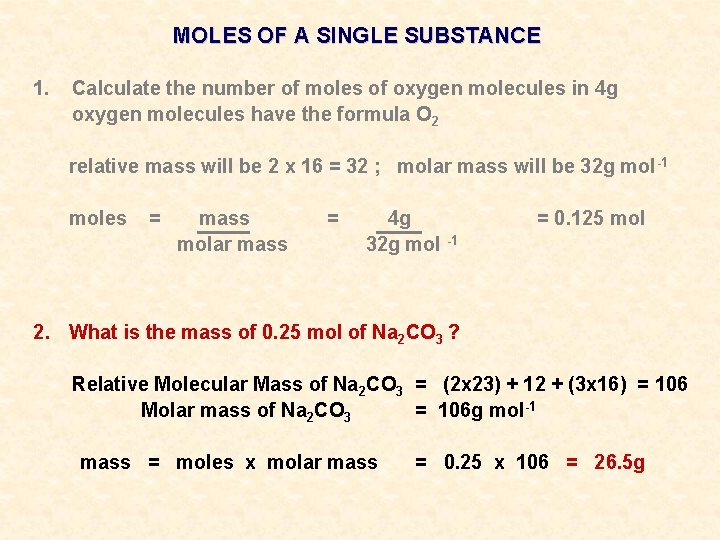

















- Slides: 62

THE MOLE A guide for A level students KNOCKHARDY PUBLISHING 2008 SPECIFICATIONS

KNOCKHARDY PUBLISHING THE MOLE INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. knockhardy. org. uk/sci. htm Navigation is achieved by using the left and right arrow keys on the keyboard

THE MOLE CONTENTS • What is a mole and why do we use it? • Calculating the number of moles of a single substance • Reacting mass calculations • Solutions and moles • Standard solutions • Volumetric calculations • Molar volume calculations

THE MOLE Before you start it would be helpful to… • know how to balance simple equations • know how to re-arrange mathematical formulae DON’T BE LEFT IN THE DARK!

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . BIG !!! 6. 022 x 1023

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . It is also known as. . . BIG !!! 6. 022 x 1023 AVOGADRO’S NUMBER It doesn’t matter what the number is as long as everybody sticks to the same value !

THE MOLE WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you. . . how many particles you get in a certain mass the mass of a certain number of particles DO I NEED TO KNOW ANYTHING ELSE ? Yes, it would help if you can balance equations AND Keep trying, you will get the idea. . . EVENTUALLY!

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value !

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value ! WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you : - how many particles you get in a certain mass the mass of a certain number of particles

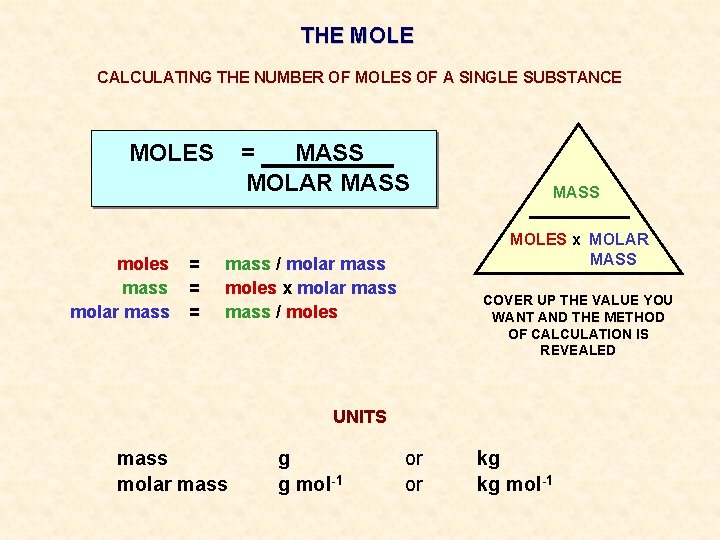
THE MOLE CALCULATING THE NUMBER OF MOLES OF A SINGLE SUBSTANCE MOLES moles mass molar mass = = MASS MOLAR MASS MOLES x MOLAR MASS mass / molar mass moles x molar mass / moles COVER UP THE VALUE YOU WANT AND THE METHOD OF CALCULATION IS REVEALED UNITS mass molar mass g g mol-1 or or kg kg mol-1

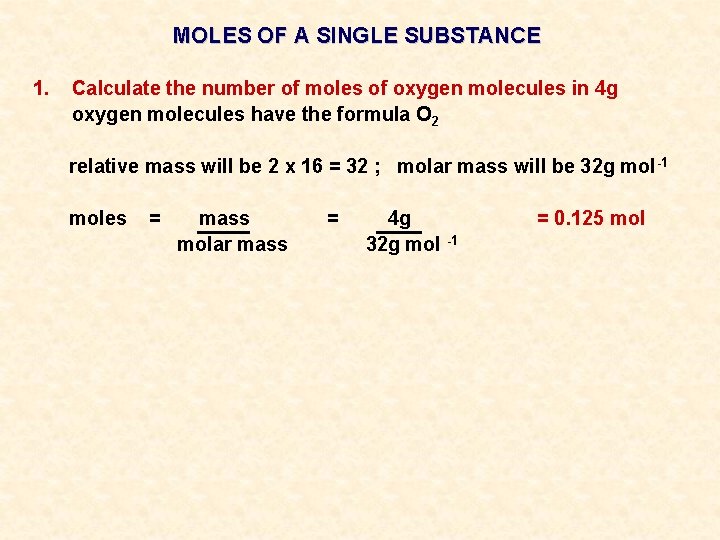
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? = 0. 125 mol
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g
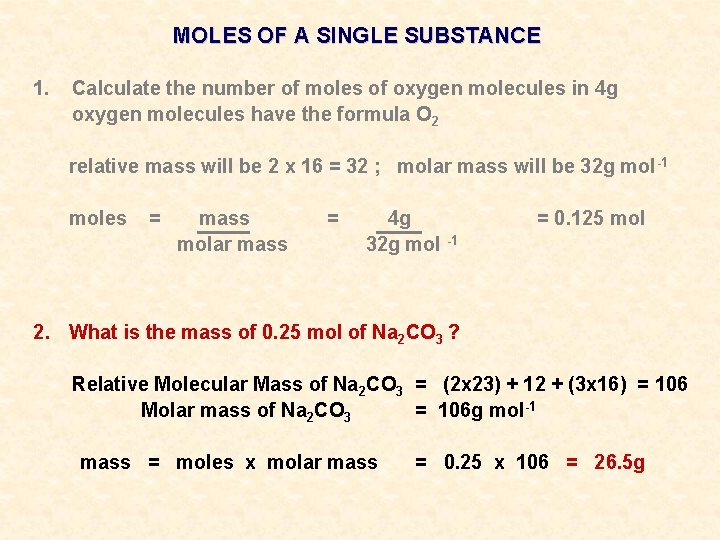
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g

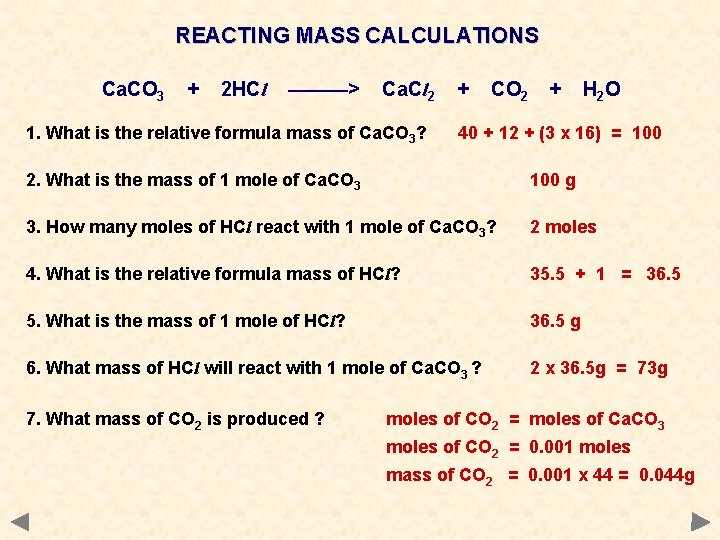
REACTING MASS CALCULATIONS Ca. CO 3 + 2 HCl ———> Ca. Cl 2 1. What is the relative formula mass of Ca. CO 3? + CO 2 + H 2 O 40 + 12 + (3 x 16) = 100 2. What is the mass of 1 mole of Ca. CO 3 100 g 3. How many moles of HCl react with 1 mole of Ca. CO 3? 2 moles 4. What is the relative formula mass of HCl? 35. 5 + 1 = 36. 5 5. What is the mass of 1 mole of HCl? 36. 5 g 6. What mass of HCl will react with 1 mole of Ca. CO 3 ? 2 x 36. 5 g = 73 g 7. What mass of CO 2 is produced ? moles of CO 2 = moles of Ca. CO 3 moles of CO 2 = 0. 001 moles mass of CO 2 = 0. 001 x 44 = 0. 044 g

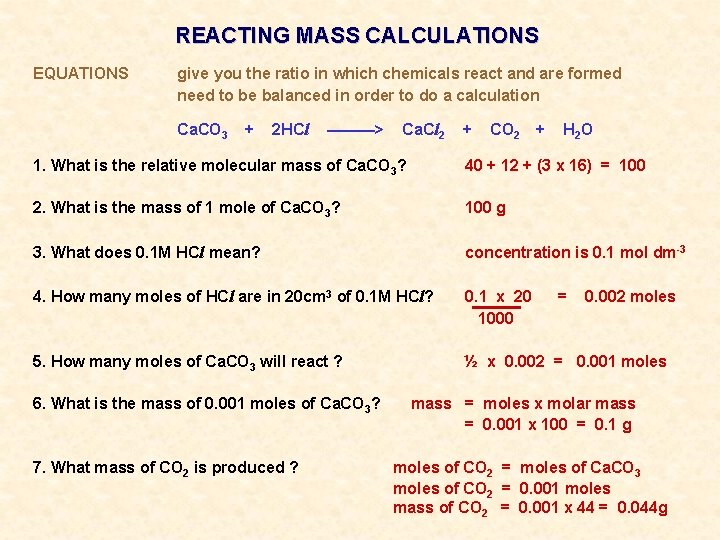
REACTING MASS CALCULATIONS EQUATIONS give you the ratio in which chemicals react and are formed need to be balanced in order to do a calculation Ca. CO 3 + 2 HCl ———> Ca. Cl 2 + CO 2 + H 2 O 1. What is the relative molecular mass of Ca. CO 3? 40 + 12 + (3 x 16) = 100 2. What is the mass of 1 mole of Ca. CO 3? 100 g 3. What does 0. 1 M HCl mean? concentration is 0. 1 mol dm-3 4. How many moles of HCl are in 20 cm 3 of 0. 1 M HCl? 0. 1 x 20 1000 5. How many moles of Ca. CO 3 will react ? ½ x 0. 002 = 0. 001 moles 6. What is the mass of 0. 001 moles of Ca. CO 3? 7. What mass of CO 2 is produced ? = 0. 002 moles mass = moles x molar mass = 0. 001 x 100 = 0. 1 g moles of CO 2 = moles of Ca. CO 3 moles of CO 2 = 0. 001 moles mass of CO 2 = 0. 001 x 44 = 0. 044 g

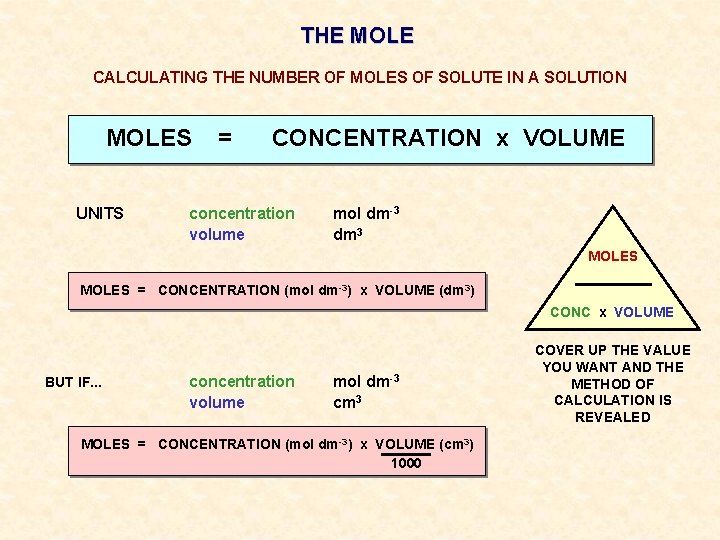
THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION MOLES UNITS = CONCENTRATION x VOLUME concentration volume mol dm-3 dm 3 MOLES = CONCENTRATION (mol dm-3) x VOLUME (dm 3) CONC x VOLUME BUT IF. . . concentration volume mol dm-3 cm 3 MOLES = CONCENTRATION (mol dm-3) x VOLUME (cm 3) 1000 COVER UP THE VALUE YOU WANT AND THE METHOD OF CALCULATION IS REVEALED

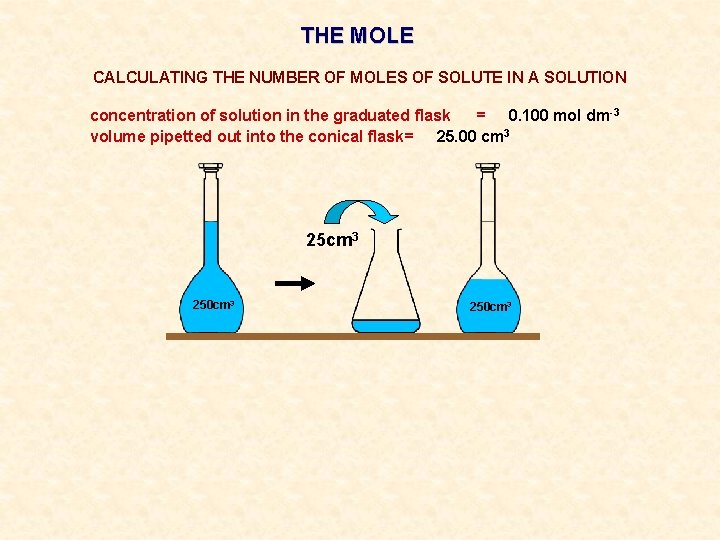
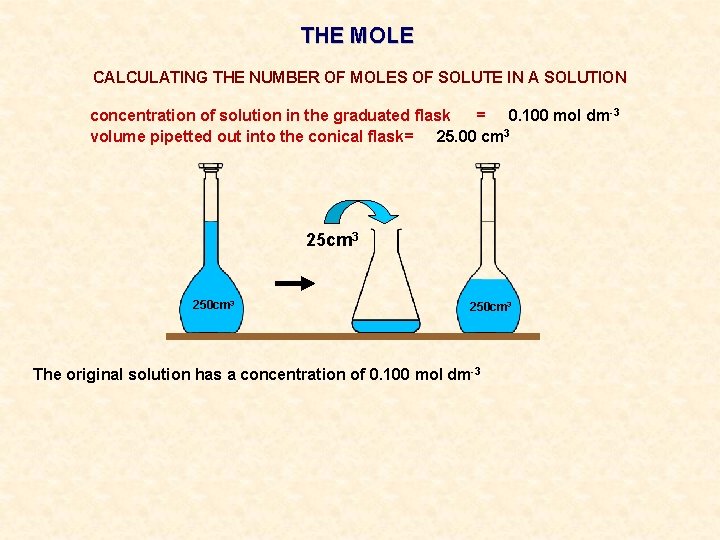
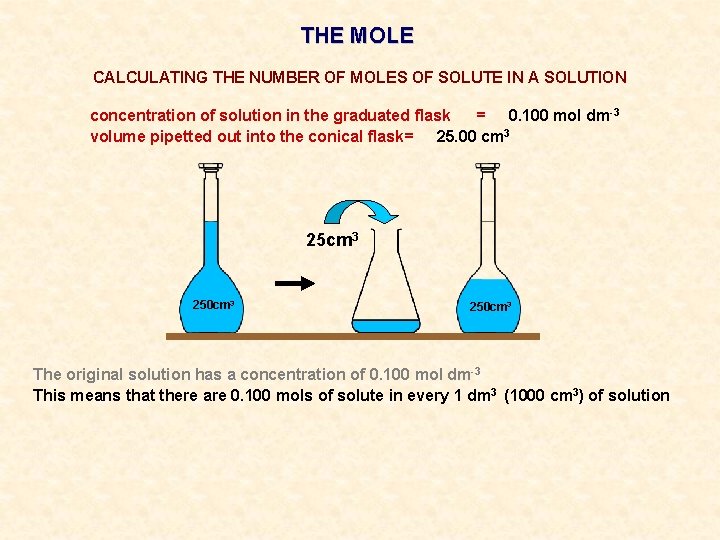
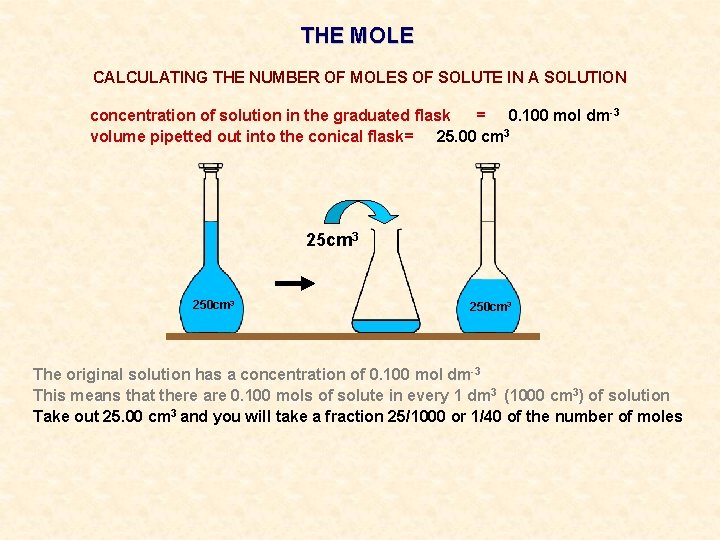
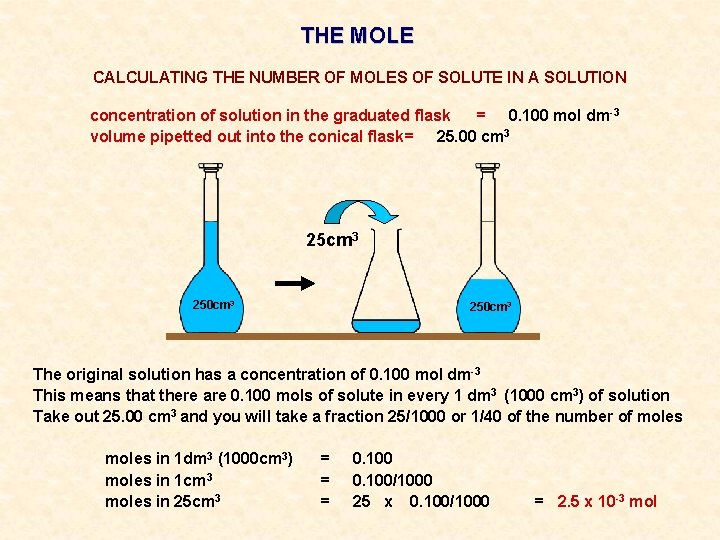
THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3

THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3 The original solution has a concentration of 0. 100 mol dm-3

THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3 The original solution has a concentration of 0. 100 mol dm-3 This means that there are 0. 100 mols of solute in every 1 dm 3 (1000 cm 3) of solution

THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3 The original solution has a concentration of 0. 100 mol dm-3 This means that there are 0. 100 mols of solute in every 1 dm 3 (1000 cm 3) of solution Take out 25. 00 cm 3 and you will take a fraction 25/1000 or 1/40 of the number of moles

THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3 The original solution has a concentration of 0. 100 mol dm-3 This means that there are 0. 100 mols of solute in every 1 dm 3 (1000 cm 3) of solution Take out 25. 00 cm 3 and you will take a fraction 25/1000 or 1/40 of the number of moles in 1 dm 3 (1000 cm 3) moles in 1 cm 3 moles in 25 cm 3 = = = 0. 100/1000 25 x 0. 100/1000 = 2. 5 x 10 -3 mol

THE MOLE CALCULATING THE NUMBER OF MOLES OF SOLUTE IN A SOLUTION concentration of solution in the graduated flask = 0. 100 mol dm-3 volume pipetted out into the conical flask= 25. 00 cm 3 250 cm 3 The original solution has a concentration of 0. 100 mol dm-3 This means that there are 0. 100 mols of solute in every 1 dm 3 (1000 cm 3) of solution Take out 25. 00 cm 3 and you will take a fraction 25/1000 or 1/40 of the number of moles in 1 dm 3 (1000 cm 3) moles in 1 cm 3 moles in 25 cm 3 = = = 0. 100/1000 25 x 0. 100/1000 = 2. 5 x 10 -3 mol

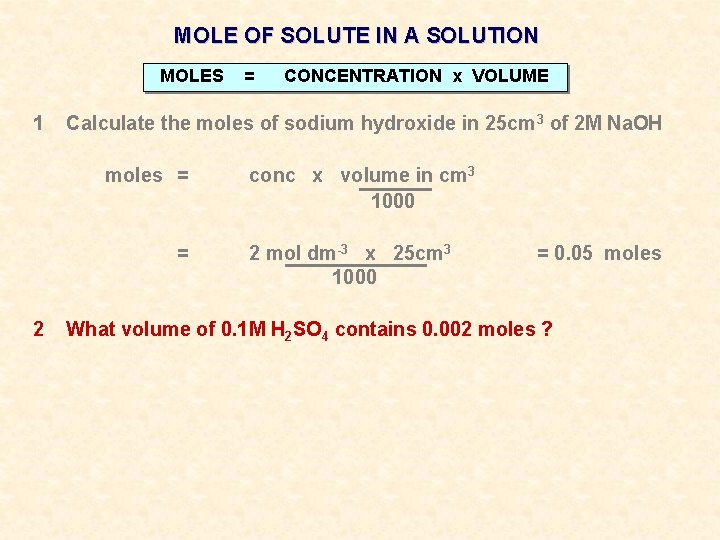
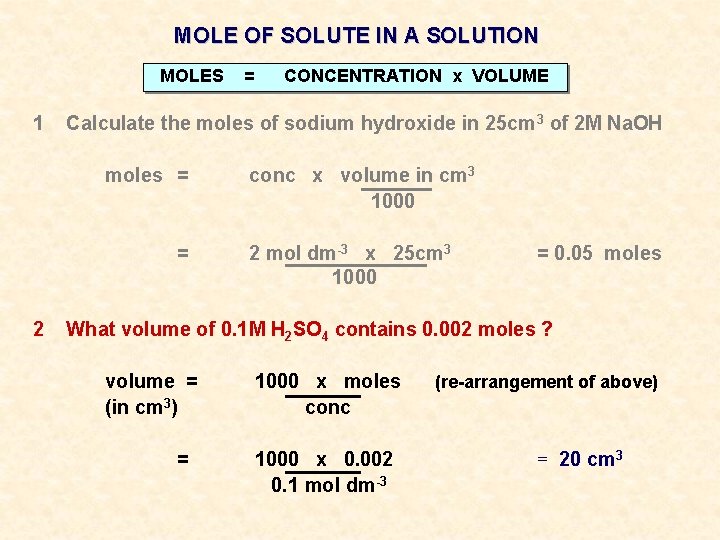
MOLE OF SOLUTE IN A SOLUTION MOLES 1 = CONCENTRATION x VOLUME Calculate the moles of sodium hydroxide in 25 cm 3 of 2 M Na. OH

MOLE OF SOLUTE IN A SOLUTION MOLES 1 = CONCENTRATION x VOLUME Calculate the moles of sodium hydroxide in 25 cm 3 of 2 M Na. OH moles = = conc x volume in cm 3 1000 2 mol dm-3 x 25 cm 3 1000 = 0. 05 moles

MOLE OF SOLUTE IN A SOLUTION MOLES 1 CONCENTRATION x VOLUME Calculate the moles of sodium hydroxide in 25 cm 3 of 2 M Na. OH moles = = 2 = conc x volume in cm 3 1000 2 mol dm-3 x 25 cm 3 1000 = 0. 05 moles What volume of 0. 1 M H 2 SO 4 contains 0. 002 moles ?

MOLE OF SOLUTE IN A SOLUTION MOLES 1 CONCENTRATION x VOLUME Calculate the moles of sodium hydroxide in 25 cm 3 of 2 M Na. OH moles = = 2 = conc x volume in cm 3 1000 2 mol dm-3 x 25 cm 3 1000 = 0. 05 moles What volume of 0. 1 M H 2 SO 4 contains 0. 002 moles ? volume = (in cm 3) = 1000 x moles conc 1000 x 0. 002 0. 1 mol dm-3 (re-arrangement of above) = 20 cm 3

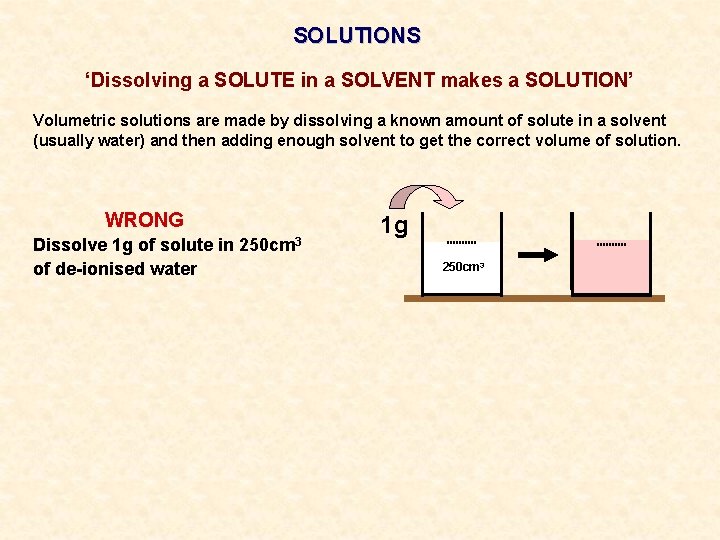
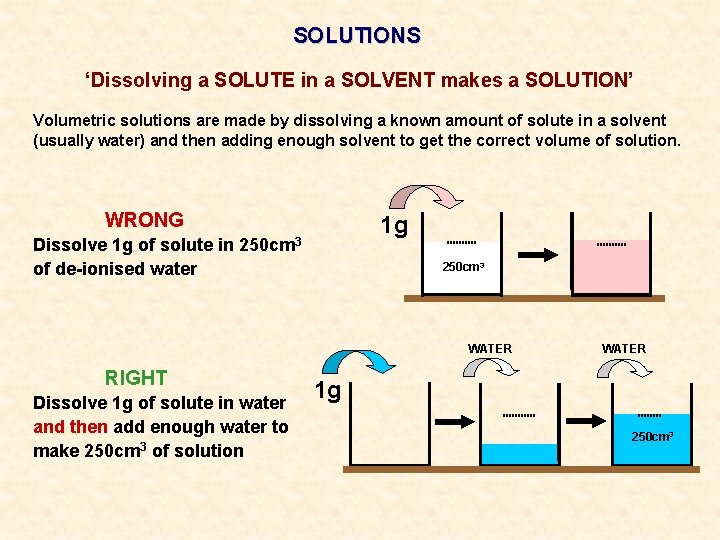
SOLUTIONS ‘Dissolving a SOLUTE in a SOLVENT makes a SOLUTION’ Volumetric solutions are made by dissolving a known amount of solute in a solvent (usually water) and then adding enough solvent to get the correct volume of solution. WRONG Dissolve 1 g of solute in of de-ionised water 250 cm 3 1 g 250 cm 3

SOLUTIONS ‘Dissolving a SOLUTE in a SOLVENT makes a SOLUTION’ Volumetric solutions are made by dissolving a known amount of solute in a solvent (usually water) and then adding enough solvent to get the correct volume of solution. WRONG Dissolve 1 g of solute in of de-ionised water 1 g 250 cm 3 WATER RIGHT Dissolve 1 g of solute in water and then add enough water to make 250 cm 3 of solution WATER 1 g 250 cm 3

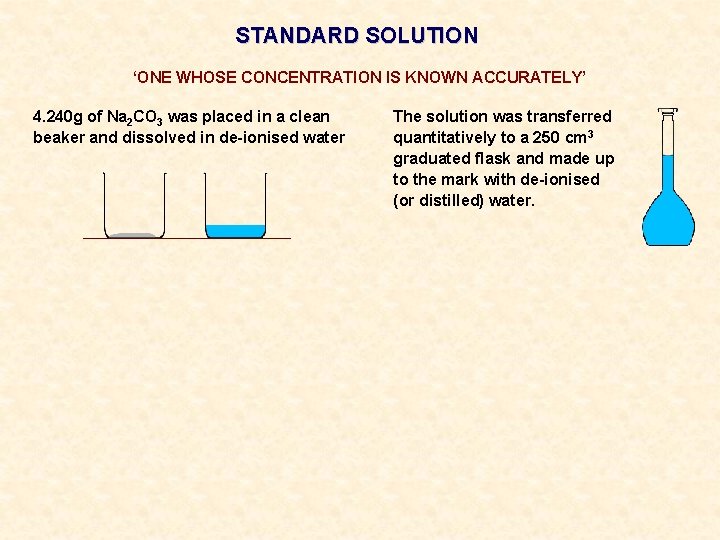
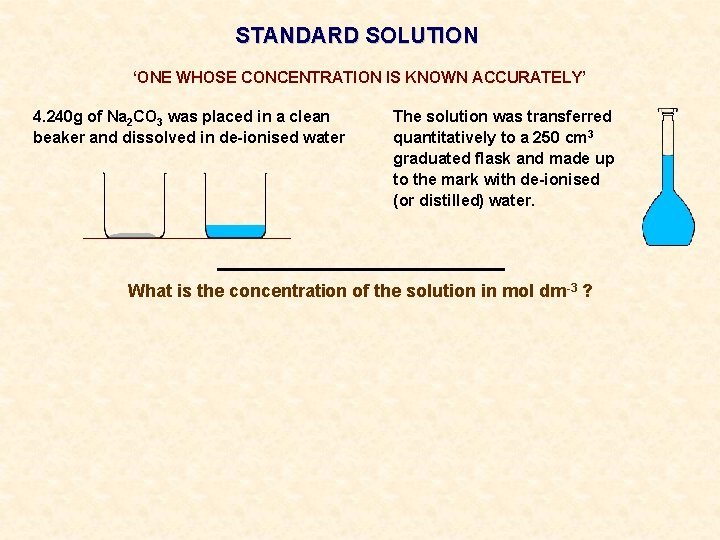
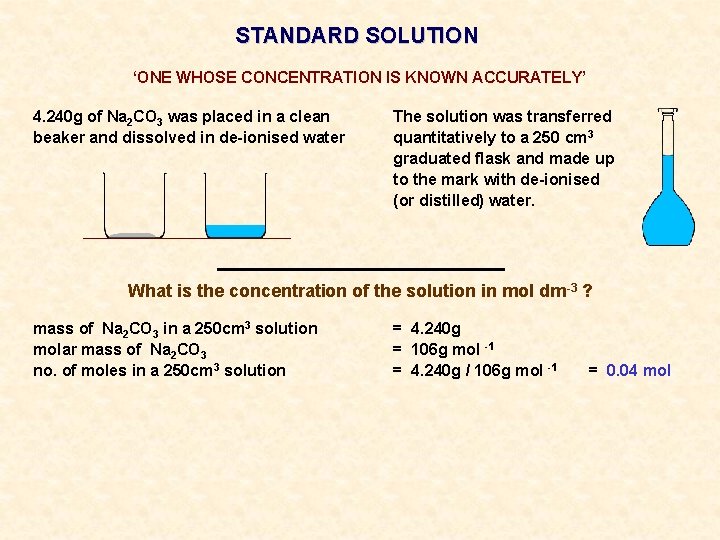
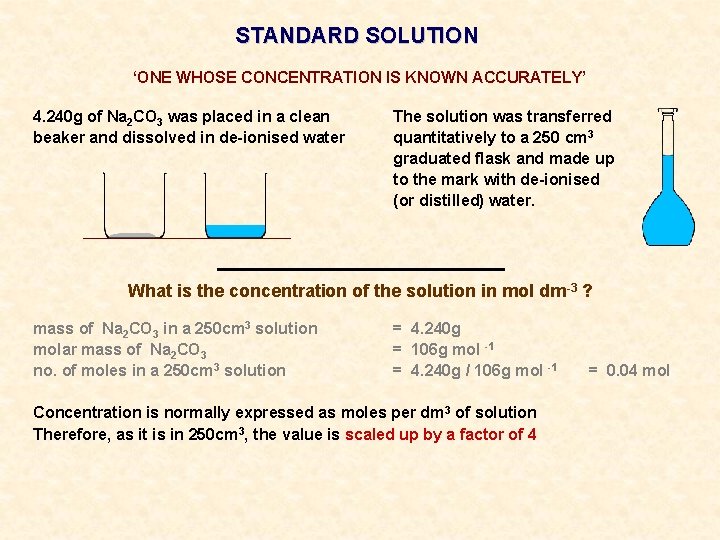
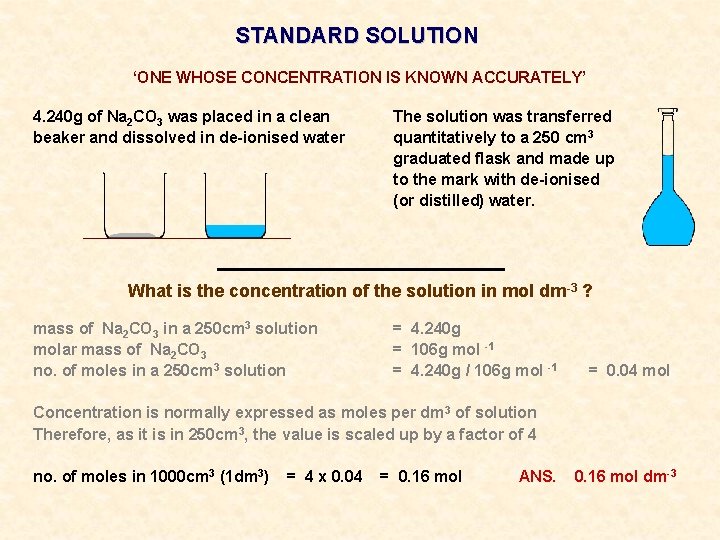
STANDARD SOLUTION ‘ONE WHOSE CONCENTRATION IS KNOWN ACCURATELY’ 4. 240 g of Na 2 CO 3 was placed in a clean beaker and dissolved in de-ionised water The solution was transferred quantitatively to a 250 cm 3 graduated flask and made up to the mark with de-ionised (or distilled) water.

STANDARD SOLUTION ‘ONE WHOSE CONCENTRATION IS KNOWN ACCURATELY’ 4. 240 g of Na 2 CO 3 was placed in a clean beaker and dissolved in de-ionised water The solution was transferred quantitatively to a 250 cm 3 graduated flask and made up to the mark with de-ionised (or distilled) water. What is the concentration of the solution in mol dm-3 ?

STANDARD SOLUTION ‘ONE WHOSE CONCENTRATION IS KNOWN ACCURATELY’ 4. 240 g of Na 2 CO 3 was placed in a clean beaker and dissolved in de-ionised water The solution was transferred quantitatively to a 250 cm 3 graduated flask and made up to the mark with de-ionised (or distilled) water. What is the concentration of the solution in mol dm-3 ? mass of Na 2 CO 3 in a 250 cm 3 solution molar mass of Na 2 CO 3 no. of moles in a 250 cm 3 solution = 4. 240 g = 106 g mol -1 = 4. 240 g / 106 g mol -1 = 0. 04 mol

STANDARD SOLUTION ‘ONE WHOSE CONCENTRATION IS KNOWN ACCURATELY’ 4. 240 g of Na 2 CO 3 was placed in a clean beaker and dissolved in de-ionised water The solution was transferred quantitatively to a 250 cm 3 graduated flask and made up to the mark with de-ionised (or distilled) water. What is the concentration of the solution in mol dm-3 ? mass of Na 2 CO 3 in a 250 cm 3 solution molar mass of Na 2 CO 3 no. of moles in a 250 cm 3 solution = 4. 240 g = 106 g mol -1 = 4. 240 g / 106 g mol -1 Concentration is normally expressed as moles per dm 3 of solution Therefore, as it is in 250 cm 3, the value is scaled up by a factor of 4 = 0. 04 mol

STANDARD SOLUTION ‘ONE WHOSE CONCENTRATION IS KNOWN ACCURATELY’ 4. 240 g of Na 2 CO 3 was placed in a clean beaker and dissolved in de-ionised water The solution was transferred quantitatively to a 250 cm 3 graduated flask and made up to the mark with de-ionised (or distilled) water. What is the concentration of the solution in mol dm-3 ? mass of Na 2 CO 3 in a 250 cm 3 solution molar mass of Na 2 CO 3 no. of moles in a 250 cm 3 solution = 4. 240 g = 106 g mol -1 = 4. 240 g / 106 g mol -1 = 0. 04 mol Concentration is normally expressed as moles per dm 3 of solution Therefore, as it is in 250 cm 3, the value is scaled up by a factor of 4 no. of moles in 1000 cm 3 (1 dm 3) = 4 x 0. 04 = 0. 16 mol ANS. 0. 16 mol dm-3

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out?

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out? What concentration is the solution to be? How many moles will be in 1 dm 3 ? How many moles will be in 250 cm 3 ? = 0. 100 mol dm-3 = 0. 100 mol = 0. 100/4 = 0. 025 mol

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out? What concentration is the solution to be? How many moles will be in 1 dm 3 ? How many moles will be in 250 cm 3 ? = 0. 100 mol dm-3 = 0. 100 mol = 0. 100/4 = 0. 025 mol What is the formula of anhydrous sodium carbonate? What is the relative formula mass? What is the molar mass? = Na 2 CO 3 = 106 g mol -1

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out? What concentration is the solution to be? How many moles will be in 1 dm 3 ? How many moles will be in 250 cm 3 ? = 0. 100 mol dm-3 = 0. 100 mol = 0. 100/4 = 0. 025 mol What is the formula of anhydrous sodium carbonate? What is the relative formula mass? What is the molar mass? = Na 2 CO 3 = 106 g mol -1 What mass of Na 2 CO 3 is in 0. 025 moles = 0. 025 x 106 = 2. 650 g of Na 2 CO 3 ? (mass = moles x molar mass)

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out? What concentration is the solution to be? How many moles will be in 1 dm 3 ? How many moles will be in 250 cm 3 ? = 0. 100 mol dm-3 = 0. 100 mol = 0. 100/4 = 0. 025 mol What is the formula of anhydrous sodium carbonate? What is the relative formula mass? What is the molar mass? = Na 2 CO 3 = 106 g mol -1 What mass of Na 2 CO 3 is in 0. 025 moles = 0. 025 x 106 = 2. 650 g of Na 2 CO 3 ? (mass = moles x molar mass) ANS. The chemist will have to weigh out 2. 650 g, dissolve it in water and then make the solution up to 250 cm 3 in a graduated flask.

STANDARD SOLUTION How to work out how much to weigh out A chemist needs to make up a 250 cm 3 standard solution of 0. 100 M sodium carbonate from anhydrous sodium carbonate. How much will they need to weigh out? What concentration is the solution to be? How many moles will be in 1 dm 3 ? How many moles will be in 250 cm 3 ? = 0. 100 mol dm-3 = 0. 100 mol = 0. 100/4 = 0. 025 mol What is the formula of anhydrous sodium carbonate? What is the relative formula mass? What is the molar mass? = Na 2 CO 3 = 106 g mol -1 What mass of Na 2 CO 3 is in 0. 025 moles = 0. 025 x 106 = 2. 650 g of Na 2 CO 3 ? (mass = moles x molar mass) ANS. The chemist will have to weigh out 2. 650 g, dissolve it in water and then make the solution up to 250 cm 3 in a graduated flask.

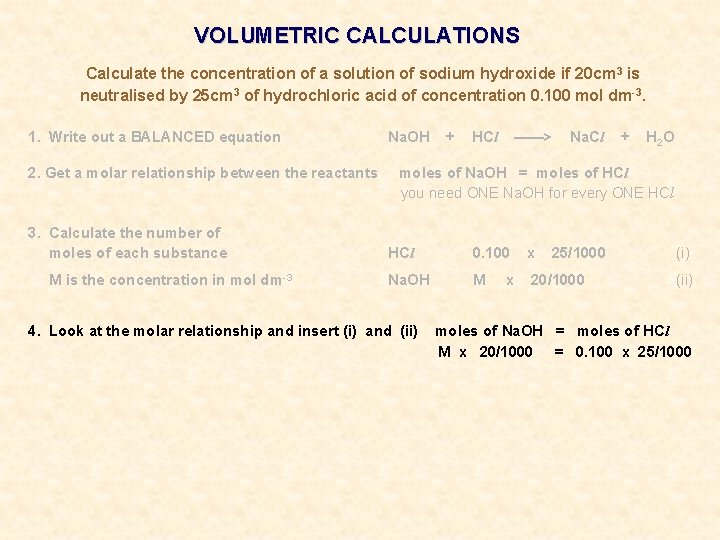
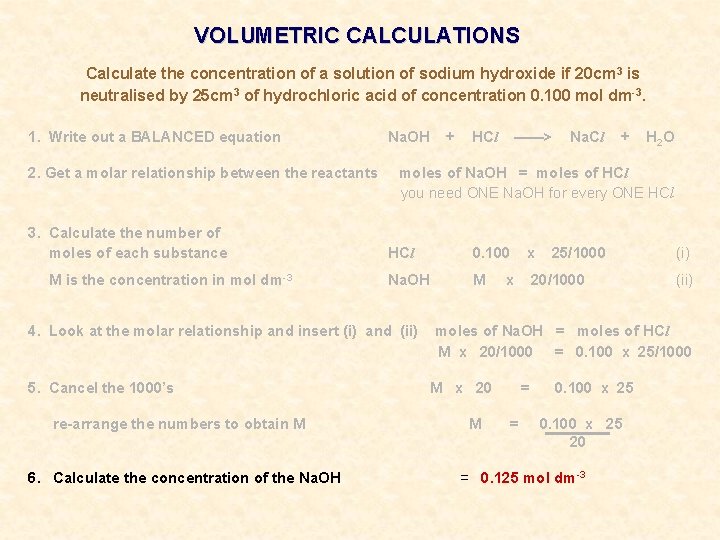
VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3.

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation Na. OH + HCl ——> Na. Cl + H 2 O

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation 2. Get a molar relationship between the reactants Na. OH + HCl ——> Na. Cl + H 2 O moles of Na. OH = moles of HCl you need ONE Na. OH for every ONE HCl

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation 2. Get a molar relationship between the reactants 3. Calculate the number of moles of each substance M is the concentration in mol dm-3 Na. OH + HCl ——> Na. Cl + H 2 O moles of Na. OH = moles of HCl you need ONE Na. OH for every ONE HCl 0. 100 x Na. OH M 20/1000 x 25/1000 (i) (ii)

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation 2. Get a molar relationship between the reactants 3. Calculate the number of moles of each substance M is the concentration in mol dm-3 Na. OH + HCl ——> Na. Cl + H 2 O moles of Na. OH = moles of HCl you need ONE Na. OH for every ONE HCl 0. 100 x Na. OH M 20/1000 4. Look at the molar relationship and insert (i) and (ii) x 25/1000 (i) (ii) moles of Na. OH = moles of HCl M x 20/1000 = 0. 100 x 25/1000

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation 2. Get a molar relationship between the reactants 3. Calculate the number of moles of each substance M is the concentration in mol dm-3 Na. OH re-arrange the numbers to obtain M HCl ——> Na. Cl + H 2 O moles of Na. OH = moles of HCl you need ONE Na. OH for every ONE HCl 0. 100 x Na. OH M 20/1000 4. Look at the molar relationship and insert (i) and (ii) 5. Cancel the 1000’s + x 25/1000 (i) (ii) moles of Na. OH = moles of HCl M x 20/1000 = 0. 100 x 25/1000 M x 20 M = = 0. 100 x 25 20

VOLUMETRIC CALCULATIONS Calculate the concentration of a solution of sodium hydroxide if 20 cm 3 is neutralised by 25 cm 3 of hydrochloric acid of concentration 0. 100 mol dm-3. 1. Write out a BALANCED equation 2. Get a molar relationship between the reactants 3. Calculate the number of moles of each substance M is the concentration in mol dm-3 Na. OH re-arrange the numbers to obtain M 6. Calculate the concentration of the Na. OH HCl ——> Na. Cl + H 2 O moles of Na. OH = moles of HCl you need ONE Na. OH for every ONE HCl 0. 100 x Na. OH M 20/1000 4. Look at the molar relationship and insert (i) and (ii) 5. Cancel the 1000’s + x 25/1000 (i) (ii) moles of Na. OH = moles of HCl M x 20/1000 = 0. 100 x 25/1000 M x 20 M = = 0. 100 x 25 20 = 0. 125 mol dm-3

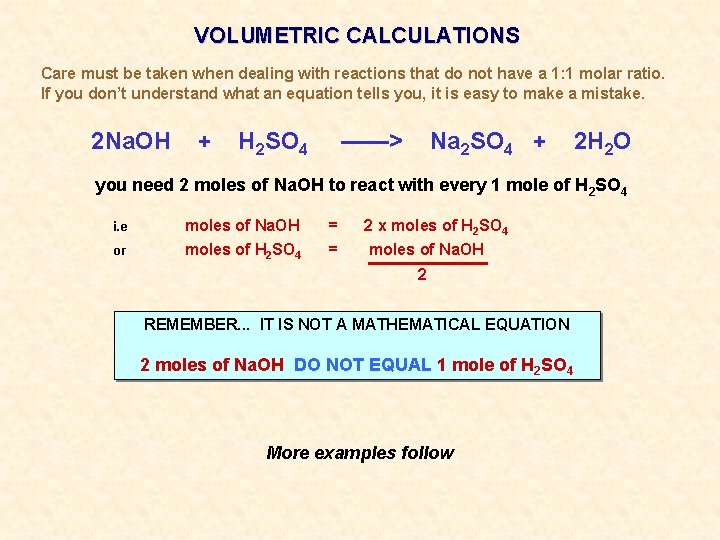
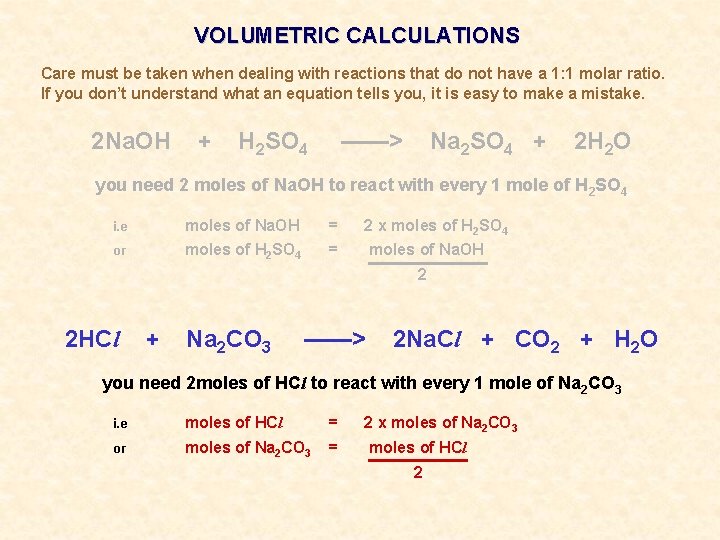
VOLUMETRIC CALCULATIONS Care must be taken when dealing with reactions that do not have a 1: 1 molar ratio. If you don’t understand what an equation tells you, it is easy to make a mistake. 2 Na. OH + H 2 SO 4 ——> Na 2 SO 4 + 2 H 2 O you need 2 moles of Na. OH to react with every 1 mole of H 2 SO 4 i. e moles of Na. OH = 2 x moles of H 2 SO 4 or moles of H 2 SO 4 = moles of Na. OH 2 REMEMBER. . . IT IS NOT A MATHEMATICAL EQUATION 2 moles of Na. OH DO NOT EQUAL 1 mole of H 2 SO 4 More examples follow

VOLUMETRIC CALCULATIONS Care must be taken when dealing with reactions that do not have a 1: 1 molar ratio. If you don’t understand what an equation tells you, it is easy to make a mistake. 2 Na. OH + H 2 SO 4 ——> Na 2 SO 4 + 2 H 2 O you need 2 moles of Na. OH to react with every 1 mole of H 2 SO 4 i. e moles of Na. OH = 2 x moles of H 2 SO 4 or moles of H 2 SO 4 = moles of Na. OH 2 2 HCl + Na 2 CO 3 ——> 2 Na. Cl + CO 2 + H 2 O you need 2 moles of HCl to react with every 1 mole of Na 2 CO 3 i. e moles of HCl = 2 x moles of Na 2 CO 3 or moles of Na 2 CO 3 = moles of HCl 2

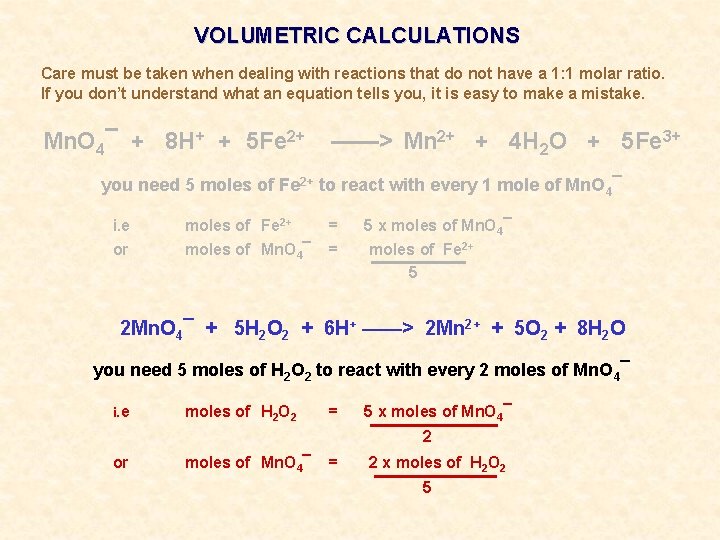
VOLUMETRIC CALCULATIONS Care must be taken when dealing with reactions that do not have a 1: 1 molar ratio. If you don’t understand what an equation tells you, it is easy to make a mistake. Mn. O 4¯ + 8 H+ + 5 Fe 2+ ——> Mn 2+ + 4 H 2 O + 5 Fe 3+ you need 5 moles of Fe 2+ to react with every 1 mole of Mn. O 4¯ i. e moles of Fe 2+ = 5 x moles of Mn. O 4¯ or moles of Mn. O 4¯ = moles of Fe 2+ 5

VOLUMETRIC CALCULATIONS Care must be taken when dealing with reactions that do not have a 1: 1 molar ratio. If you don’t understand what an equation tells you, it is easy to make a mistake. Mn. O 4¯ + 8 H+ + 5 Fe 2+ ——> Mn 2+ + 4 H 2 O + 5 Fe 3+ you need 5 moles of Fe 2+ to react with every 1 mole of Mn. O 4¯ i. e moles of Fe 2+ = 5 x moles of Mn. O 4¯ or moles of Mn. O 4¯ = moles of Fe 2+ 5 2 Mn. O 4¯ + 5 H 2 O 2 + 6 H+ ——> 2 Mn 2+ + 5 O 2 + 8 H 2 O you need 5 moles of H 2 O 2 to react with every 2 moles of Mn. O 4¯ i. e moles of H 2 O 2 = 5 x moles of Mn. O 4¯ 2 or moles of Mn. O 4¯ = 2 x moles of H 2 O 2 5

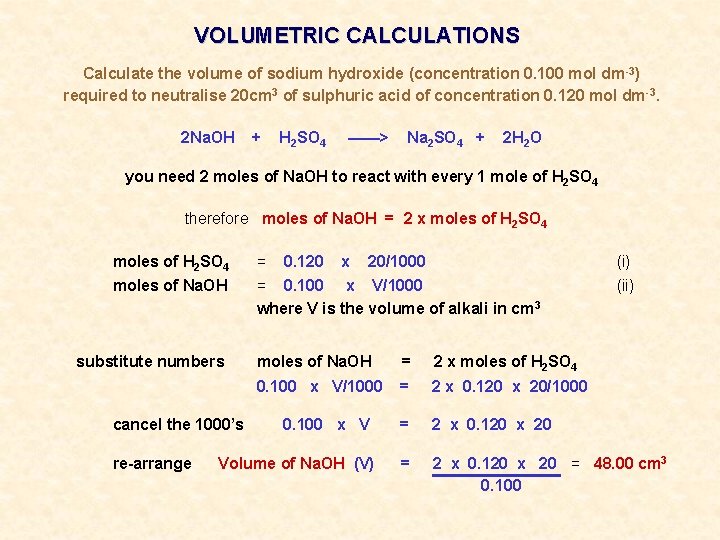
VOLUMETRIC CALCULATIONS Calculate the volume of sodium hydroxide (concentration 0. 100 mol dm -3) required to neutralise 20 cm 3 of sulphuric acid of concentration 0. 120 mol dm-3. 2 Na. OH + H 2 SO 4 ——> Na 2 SO 4 + 2 H 2 O you need 2 moles of Na. OH to react with every 1 mole of H 2 SO 4 therefore moles of Na. OH = 2 x moles of H 2 SO 4 = 0. 120 x 20/1000 (i) moles of Na. OH = 0. 100 x V/1000 where V is the volume of alkali in cm 3 (ii)

VOLUMETRIC CALCULATIONS Calculate the volume of sodium hydroxide (concentration 0. 100 mol dm -3) required to neutralise 20 cm 3 of sulphuric acid of concentration 0. 120 mol dm-3. 2 Na. OH + H 2 SO 4 ——> Na 2 SO 4 + 2 H 2 O you need 2 moles of Na. OH to react with every 1 mole of H 2 SO 4 therefore moles of Na. OH = 2 x moles of H 2 SO 4 = 20/1000 (i) moles of Na. OH = 0. 100 x V/1000 where V is the volume of alkali in cm 3 (ii) substitute numbers cancel the 1000’s re-arrange 0. 120 x moles of Na. OH = 2 x moles of H 2 SO 4 0. 100 x V/1000 = 2 x 0. 120 x 20 = 48. 00 cm 3 0. 100 x V Volume of Na. OH (V)

MOLAR VOLUME ONE MOLE OF ANY GAS OR VAPOUR OCCUPIES 22. 4 dm 3 at stp 1. Calculate the volume occupied by 0. 25 mols of carbon dioxide at stp 1 mol of carbon dioxide will occupy a volume of 22. 4 dm 3 at stp 0. 25 mol of carbon dioxide will occupy a volume of 22. 4 x 0. 25 dm 3 at stp 0. 25 mol of carbon dioxide will occupy a volume of 5. 6 dm 3 at stp = standard temperature and pressure (273 K and 105 Pa) ONE MOLE OF ANY GAS OR VAPOUR OCCUPIES 24 dm 3 at room temperature and pressure

MOLAR VOLUME ONE MOLE OF ANY GAS OR VAPOUR OCCUPIES 22. 4 dm 3 at stp 1. Calculate the volume occupied by 0. 25 mols of carbon dioxide at stp 1 mol of carbon dioxide will occupy a volume of 22. 4 dm 3 at stp 0. 25 mol of carbon dioxide will occupy a volume of 22. 4 x 0. 25 dm 3 at stp 0. 25 mol of carbon dioxide will occupy a volume of 5. 6 dm 3 at stp 2. Calculate the volume occupied by 0. 08 g of methane (CH 4) at stp Relative Molecular Mass of CH 4 = 12 + (4 x 1) Molar Mass of CH 4 = 16 g mol-1 Moles = mass/molar mass 0. 08 g / 16 g mol-1 = = 16 0. 005 mols 1 mol of methane will occupy a volume of 22. 4 dm 3 at stp 0. 005 mol of carbon dioxide will occupy a volume of 22. 4 x 0. 005 dm 3 at stp 0. 005 mol of carbon dioxide will occupy a volume of 0. 112 dm 3 at stp = standard temperature and pressure (273 K and 105 Pa) ONE MOLE OF ANY GAS OR VAPOUR OCCUPIES 24 dm 3 at room temperature and pressure

THE MOLE THE END © 2010 JONATHAN HOPTON & KNOCKHARDY PUBLISHING