Statistics for AFP Dr Mohammad A Fallaha AFP







- Slides: 11

Statistics for AFP Dr Mohammad A. Fallaha AFP in Surgery, North West Thames Honorary Clinical Research Fellow, Imperial College London

Learning Objectives • To be able to define common medical statistical terminology, including p-value, confidence interval, relative risk, hazard ratio, odds ratio, incidence, and prevalence • To be able to apply this knowledge to interpret medical statistics • To feel confident presenting a paper’s statistics

Structure 1) 2) 3) 4) Key definitions Types of analysis Statistical tests – very brief! Worked examples For MEDICS not statisticians Will be slow • Firm grasp basic concepts > tenuous grasp of complex waffle

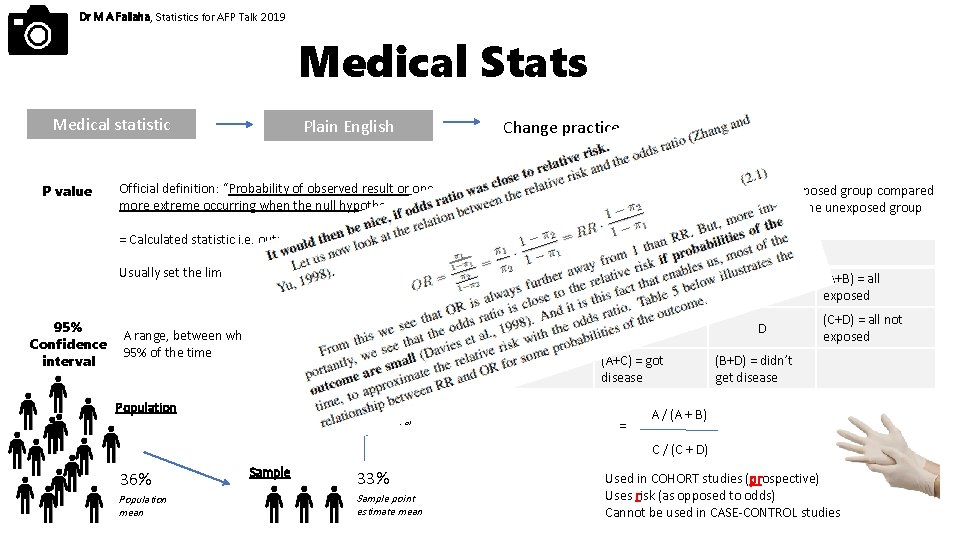
Dr M A Fallaha, Statistics for AFP Talk 2019 Medical Stats Medical statistic P value Plain English Official definition: “Probability of observed result or one more extreme occurring when the null hypothesis is true” Change practice Relative risk = Calculated statistic i. e. output Usually set the limit of significance at P = 0. 05 95% Confidence interval A range, between which the population mean value will lie 95% of the time Risk of developing disease in the exposed group compared to the risk of developing disease in the unexposed group Got disease Un-diseased Exposed A B (A+B) = all exposed Not exposed C D (C+D) = all not exposed (A+C) = got disease 30 - 36% Population Confidence interval = (B+D) = didn’t get disease A / (A + B) C / (C + D) 36% Population mean Sample 33% Sample point estimate mean Used in COHORT studies (prospective) Uses risk (as opposed to odds) Cannot be used in CASE-CONTROL studies

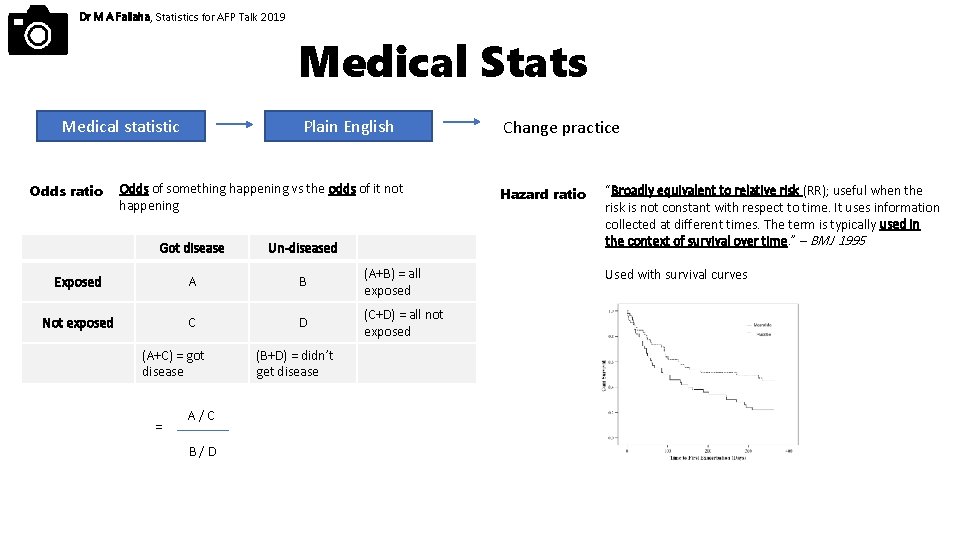
Dr M A Fallaha, Statistics for AFP Talk 2019 Medical Stats Medical statistic Odds ratio Plain English Odds of something happening vs the odds of it not happening Got disease Un-diseased Exposed A B (A+B) = all exposed Not exposed C D (C+D) = all not exposed (A+C) = got disease = A/C B/D (B+D) = didn’t get disease Change practice Hazard ratio “Broadly equivalent to relative risk (RR); useful when the risk is not constant with respect to time. It uses information collected at different times. The term is typically used in the context of survival over time. ” – BMJ 1995 Used with survival curves

Plain English Translation Relative risk e. g. prospective cohort study, RR = 1. 45 “There is a 45% increased risk of developing disease X in the group that drank coffee compared to the group that didn’t drink coffee. ” Odds ratio e. g. retrospective observational study OR = 1. 60 “The odds of chewing tobacco was 60% higher in those who had disease X compared to those who didn’t have the disease”. Hazard ratio e. g. prospective RCT, HR = 0. 79 “Over the course of ten years, those who received the drug were 21% less likely to die than those who received placebo. ”

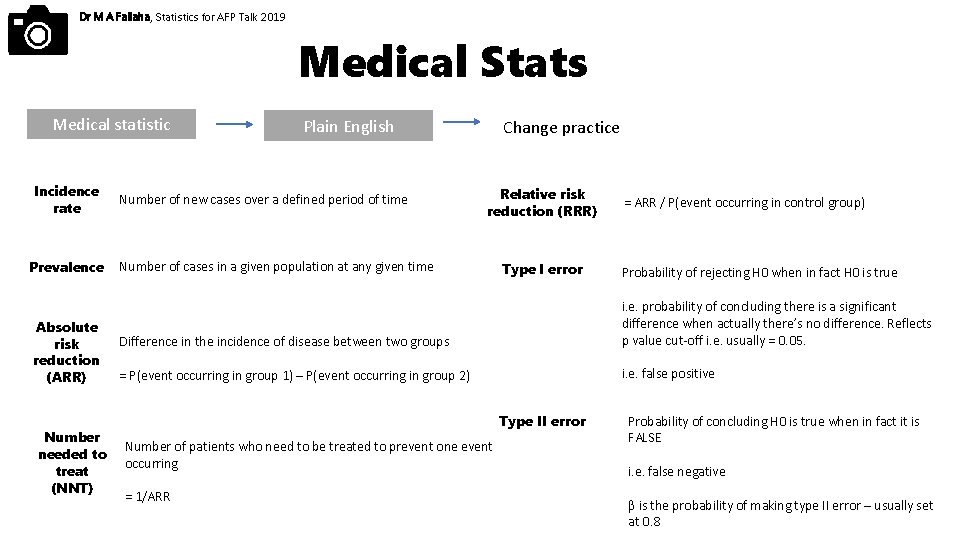
Dr M A Fallaha, Statistics for AFP Talk 2019 Medical Stats Medical statistic Incidence rate Prevalence Absolute risk reduction (ARR) Number needed to treat (NNT) Plain English Number of new cases over a defined period of time Change practice Relative risk reduction (RRR) Number of cases in a given population at any given time Type I error = ARR / P(event occurring in control group) Probability of rejecting H 0 when in fact H 0 is true Difference in the incidence of disease between two groups i. e. probability of concluding there is a significant difference when actually there’s no difference. Reflects p value cut-off i. e. usually = 0. 05. = P(event occurring in group 1) – P(event occurring in group 2) i. e. false positive Type II error Number of patients who need to be treated to prevent one event occurring = 1/ARR Probability of concluding H 0 is true when in fact it is FALSE i. e. false negative β is the probability of making type II error – usually set at 0. 8

Designing a study Power is the ability of a study to pick up a difference, when it actually exists. “The probability that a type II error will not be made in the study. ” Increase power (+ therefore ↓ β) by: 1) Increasing sample size 2) Increasing the expected effect size 3) Increasing precision of measurement

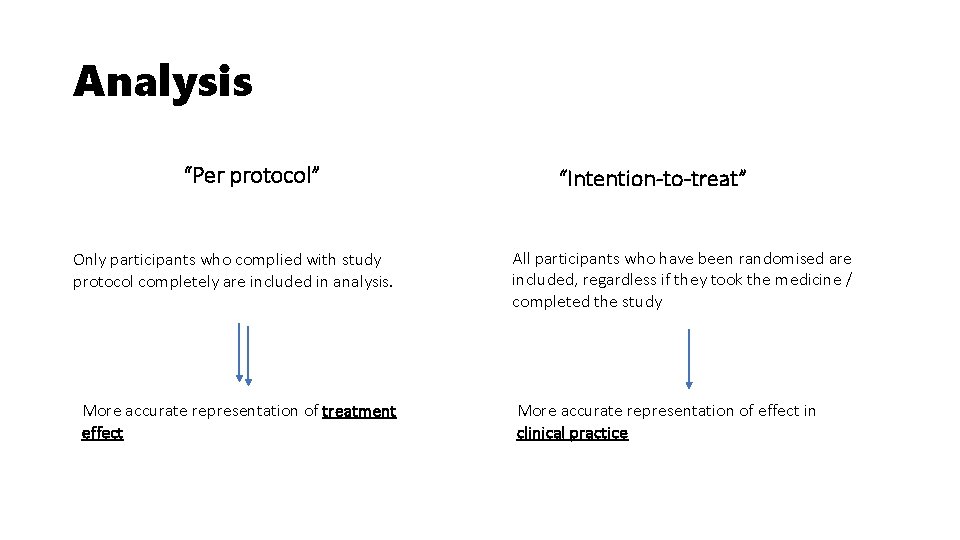
Analysis “Per protocol” Only participants who complied with study protocol completely are included in analysis. More accurate representation of treatment effect “Intention-to-treat” All participants who have been randomised are included, regardless if they took the medicine / completed the study More accurate representation of effect in clinical practice

Dr M A Fallaha, Statistics for AFP Talk 2019 How I like to approach stats 1) Find the critical line: 32 (6%) of 533 evaluable patients in the standard shunt group had a shunt revision for infection, compared with 12 (2%) of 535 evaluable patients in the antibiotic shunt group (cause-specific hazard ratio [cs. HR] 0· 38, 97· 5% CI 0· 18– 0· 80, p=0· 0038). 2) Is it significant? P value Confidence interval 3) What’s the stat? Hazard ratio 4) Convert to plain-text “There is a significant reduction of revisions for shunt infection of 62% when antibiotic shunts are used compared to standard shunts. ”

Statistics for AFP Dr Mohammad A. Fallaha AFP in Surgery, North West Thames Honorary Clinical Research Fellow, Imperial College London