Quantum Mechanics What is Quantum Physics Quantum physics













- Slides: 21

Quantum Mechanics

What is Quantum Physics? • Quantum physics takes into account every possible outcome of measurement of physical properties Quantum mechanics uses the language of PROBABILITY theory (random chance) – Dr. Farnsworth Explains – So anything can happen for no reason? – For large objects (ones we can measure), the large number of particles rule out the unlikely scenarios, and we can predict exactly what will happen. • Quantum mechanics is used to explain microscopic phenomena such as photon-atom scattering and flow of the electrons in a semiconductor; where random chances rules out over common sense.

How did we get to Quantum Mechanics? • Scientists noticed a problem with a scientific observation compared to the expected results from Newtonian Physics. • The story of Blackbody Radiation and the Ultraviolet Catastrophe:

Blackbody Radiation • Known since centuries that when a material is heated, it radiates heat and its color depends on its temperature • Example: heating elements of a stove: – Dark red: 550ºC – Bright red: 700ºC – Then: orange, yellow and finally white (really hot !) • Blackbody Radiation describes what color you will see the brightest based on temperature.

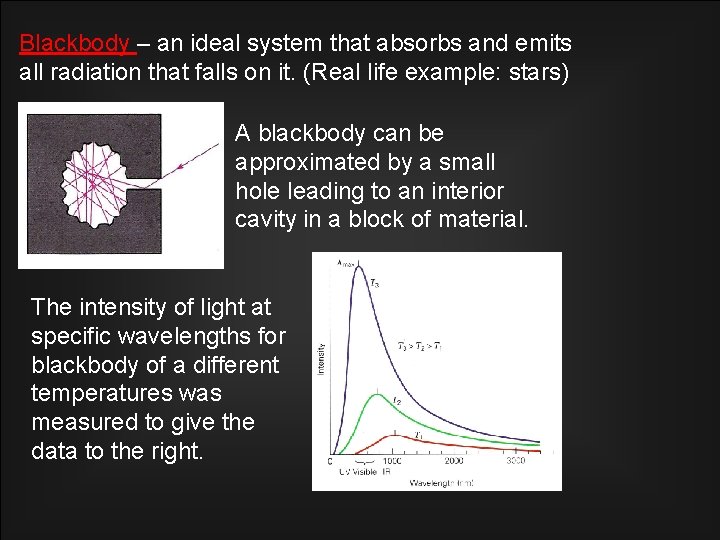
Blackbody – an ideal system that absorbs and emits all radiation that falls on it. (Real life example: stars) A blackbody can be approximated by a small hole leading to an interior cavity in a block of material. The intensity of light at specific wavelengths for blackbody of a different temperatures was measured to give the data to the right.

The Ultraviolet Catastrophe The problem is the expected data from Newtonian Physics looks like this: This means the intensity would have to be HUGE as the wavelength became ultraviolet, but it was actually smaller.

Quantization of Energy Max Planck successfully explained the spectrum of blackbody radiation by proposing a radical hypothesis. According to Planck’s hypothesis, the energy of the oscillating atoms emitting the radiation have only discrete, or particular, amounts of energy rather than a continuous distribution of energies. The energy is: E = hf E = energy quantum of light h = Planck’s constant (6. 63 x 10 -34 J·s) f = frequency of light

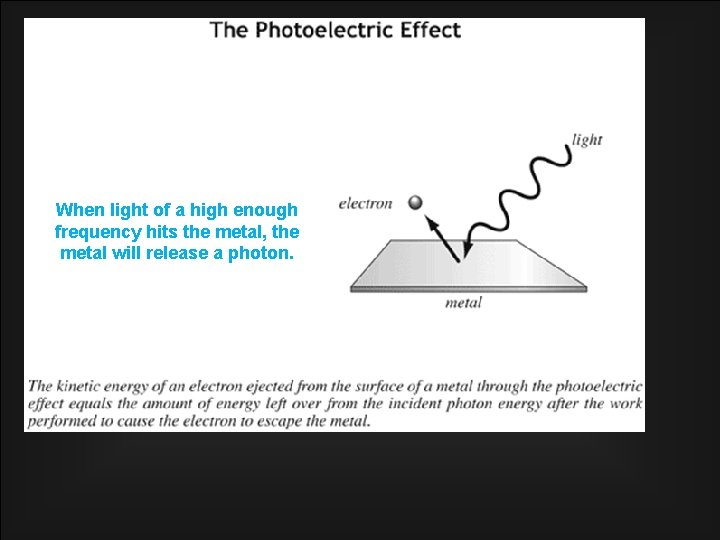
Quantization of Light • Scientists did NOT like the idea of energy quantization, because it meant that Newtonian Physics was wrong • Planck’s findings were not widely accepted until Albert Einstein proved it experimentally in 1905 with the Photoelectric Effect: When light shines on any metal surface, the surface can release electrons only if the frequency of the light is high enough. This means energy of light depends on the frequency of the light, not the intensity of the light. Einstein found that the energy of light must be quantized for the to work… E = hf

When light of a high enough frequency hits the metal, the metal will release a photon.

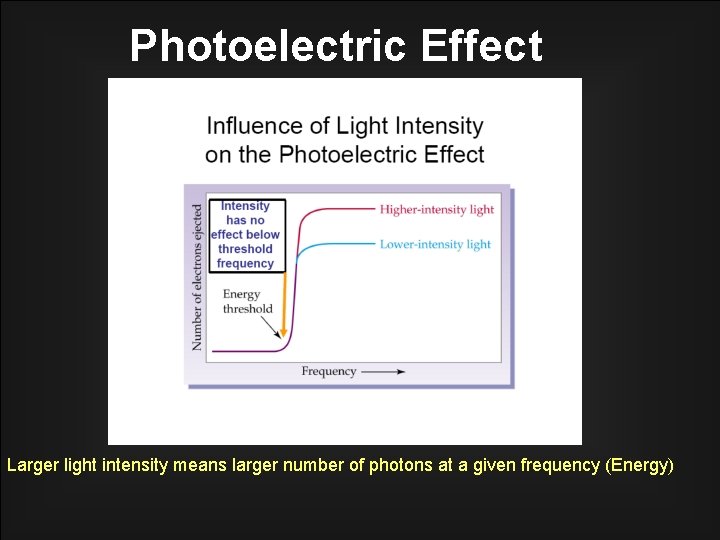
Photoelectric Effect Larger light intensity means larger number of photons at a given frequency (Energy)

Consequences of the Photoelectric Effect – – – The photoelectric effect provides evidence for the particle nature of light. Photons. Frequency is the energy of light quanta. If light shines on the surface of a metal, there is a point at which electrons are ejected from the metal. The electrons will only be ejected once threshold frequency is reached. The intensity of light increased the number of electrons released, so intensity is the amount of light quanta.

Wave Particle Duality • Scientists finally had to accept that light was a wave and a particle. • Typically, light will only behave as a light OR a particle at a single time. • Scientists were finally able to photograph light behaving as both a wave an a particle at the same time in 2015! • But if light is a wave and a particle, that leaves the possibility that particles could be waves! • Double Slit Experiment and Quantum Weirdness • Does this mean everything is a wave and particle? • Remember Quantum mechanics is meant only for microscopic phenomena. • But it does work for ALL phenomena! This is why quantum mechanics all depends on probability.

Schrödinger’s Cat • The current understanding of waveparticle duality, is that all things are particles and waves at the same time. • This has some strange consequences: – Schrödinger's Cat – Real life application according to the “Big Bang Theory” • This brings about the beginning of the Parallel Universe theories of physics.


Wave Particle Duality • Einstein was not comfortable with the consequences of quantum theory acting completely by probability and argued with Max Planck, saying: “I am convinced that God does not play dice” • Planck’s response was: “Do not tell God how to run the Universe”

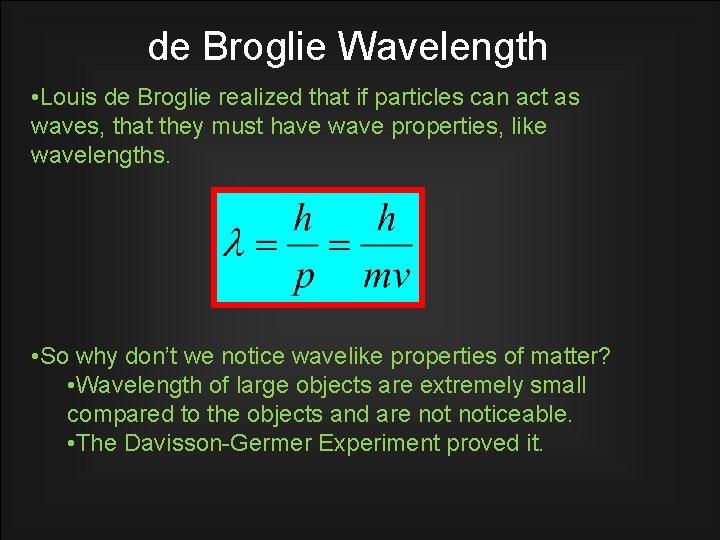
de Broglie Wavelength • Louis de Broglie realized that if particles can act as waves, that they must have wave properties, like wavelengths. • So why don’t we notice wavelike properties of matter? • Wavelength of large objects are extremely small compared to the objects and are noticeable. • The Davisson-Germer Experiment proved it.

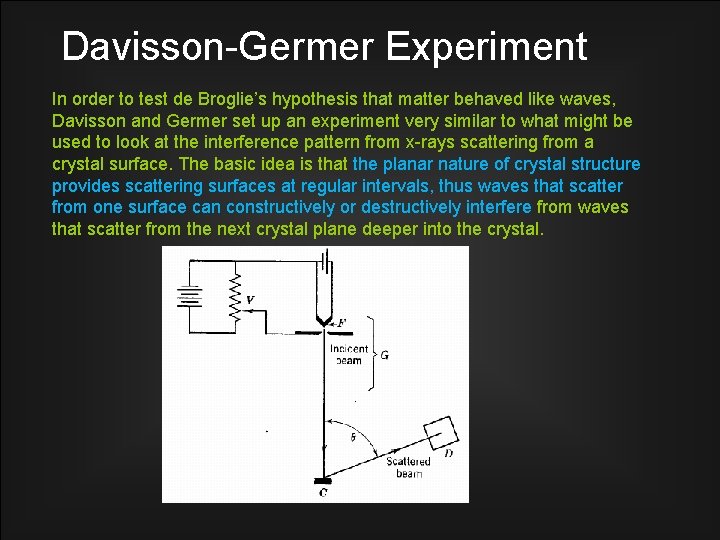
Davisson-Germer Experiment In order to test de Broglie’s hypothesis that matter behaved like waves, Davisson and Germer set up an experiment very similar to what might be used to look at the interference pattern from x-rays scattering from a crystal surface. The basic idea is that the planar nature of crystal structure provides scattering surfaces at regular intervals, thus waves that scatter from one surface can constructively or destructively interfere from waves that scatter from the next crystal plane deeper into the crystal.

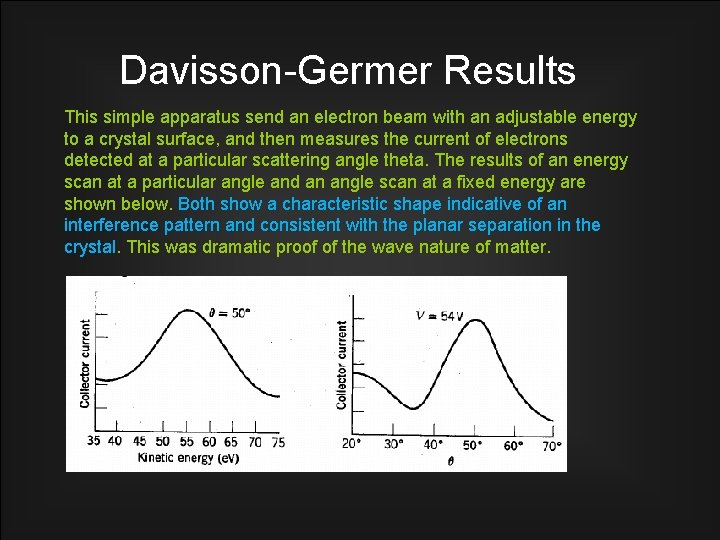
Davisson-Germer Results This simple apparatus send an electron beam with an adjustable energy to a crystal surface, and then measures the current of electrons detected at a particular scattering angle theta. The results of an energy scan at a particular angle and an angle scan at a fixed energy are shown below. Both show a characteristic shape indicative of an interference pattern and consistent with the planar separation in the crystal. This was dramatic proof of the wave nature of matter.

Heisenberg Uncertainty Principle • Wave-particle duality means we only know probabilities of the location of our particles when we measure their location. • We could find the ‘location’ of the particle by looking at a specific part of the wave • But then we can’t see the rest of the wave and have no idea where it is traveling. • The more we know about location the less we know about its speed. • We could find the ‘motion’ of the particle by looking at the entire wave. • But then we have no idea where the particle is on the wave. • It could even be places it cannot possibly be.

Heisenberg Uncertainty Principle: You cannot simultaneously know the location and momentum of a particle. • The more you know about the location, the less you can know about the momentum. • This quantum realization of wave-particle duality completely changes our classical understanding of the structure of the atom.

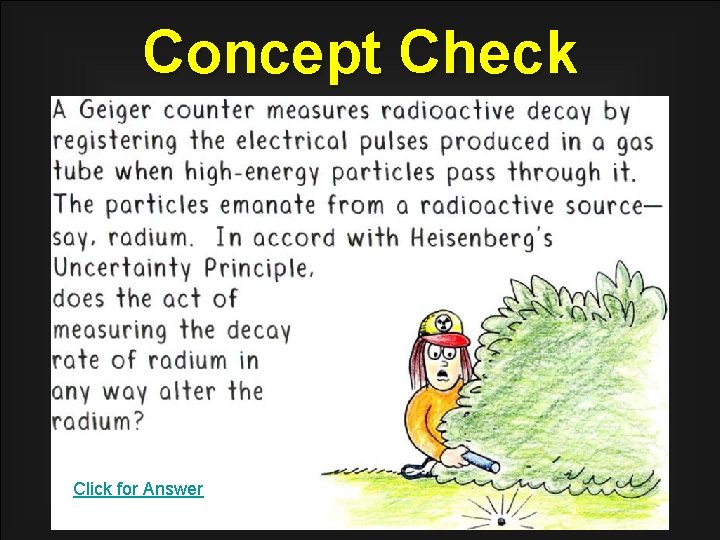
Concept Check Click for Answer