Quantum Mechanics A photon checks into a hotel



















- Slides: 41

Quantum Mechanics

A photon checks into a hotel and the bell hop asks, “Can I help you with your luggage? ” The photon replies, “I don’t have any. I’m traveling light. ”

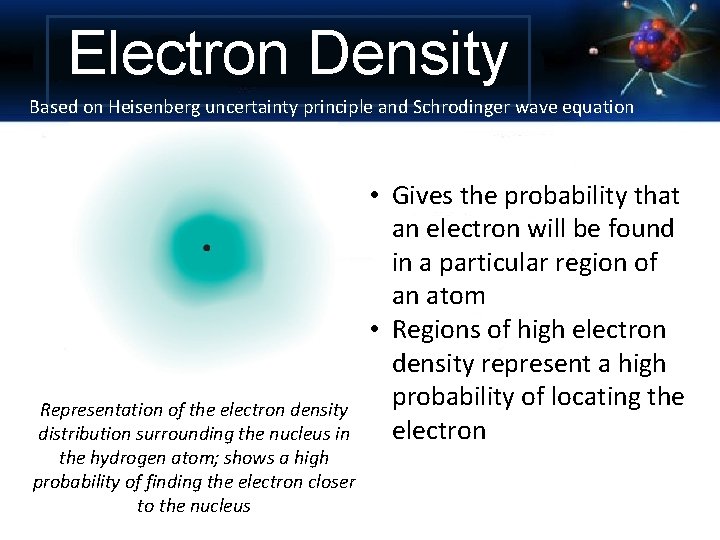
Electron Density Based on Heisenberg uncertainty principle and Schrodinger wave equation Representation of the electron density distribution surrounding the nucleus in the hydrogen atom; shows a high probability of finding the electron closer to the nucleus • Gives the probability that an electron will be found in a particular region of an atom • Regions of high electron density represent a high probability of locating the electron

Atomic Orbital • Way to distinguish Bohr’s model from the current quantum mechanical model • Probability of locating the electron in 3 D space around the nucleus • Has a characteristic energy

Quantum numbers used to describe atomic orbitals and to label electrons that reside in them • Principle quantum number (n) • Angular momentum quantum number • Magnetic quantum number • Electron spin quantum number

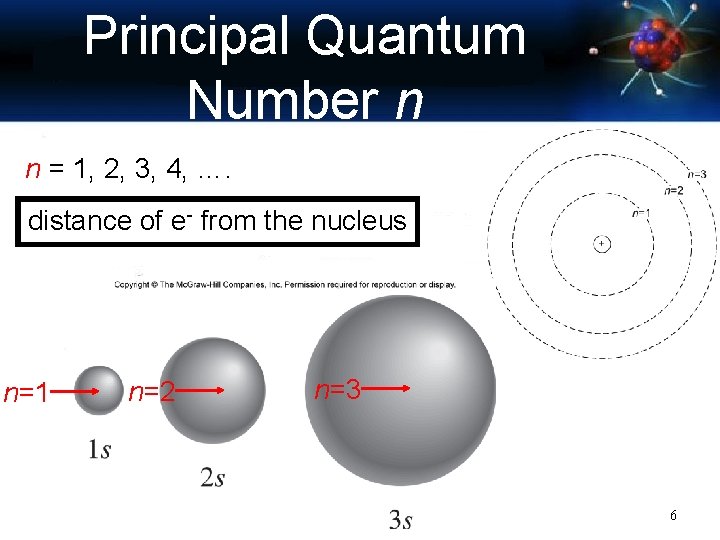
Principal Quantum Number n n = 1, 2, 3, 4, …. distance of e- from the nucleus n=1 n=2 n=3 6

Energy levels are like rungs of a ladder. You cannot be in between a rung Energy levels in an atom’s electron are unequally spaced. The higher energy levels are closer together.

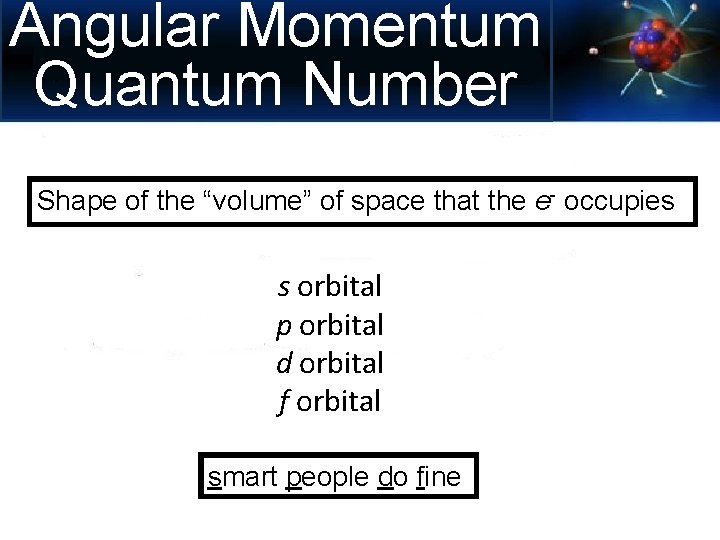
Angular Momentum Quantum Number Shape of the “volume” of space that the e- occupies s orbital p orbital d orbital f orbital smart people do fine

Magnetic Quantum Number • Describes the orientation of the orbitals in space • All orientations are identical in energy

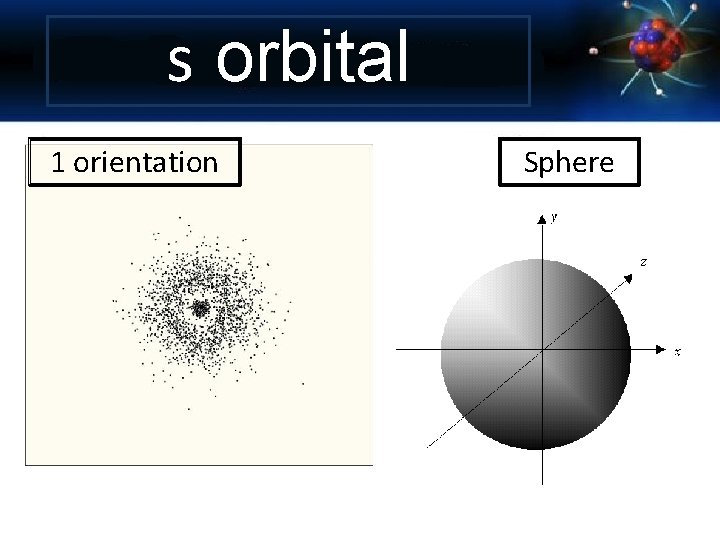
s orbital 1 orientation Sphere

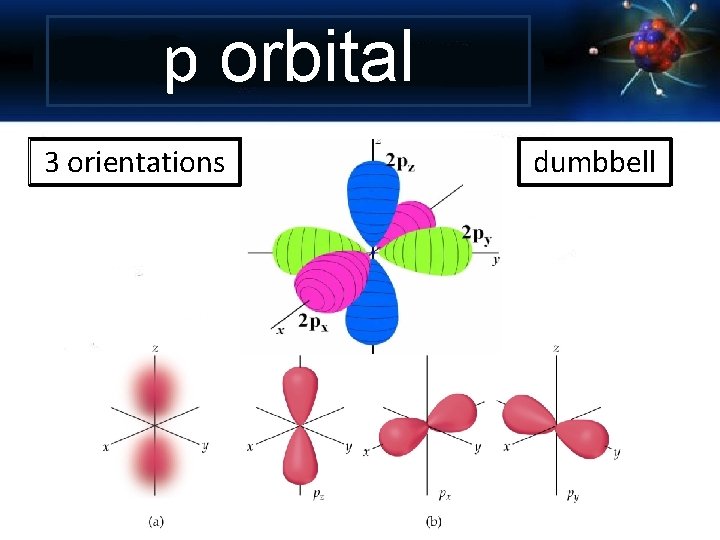
p orbital 3 orientations dumbbell

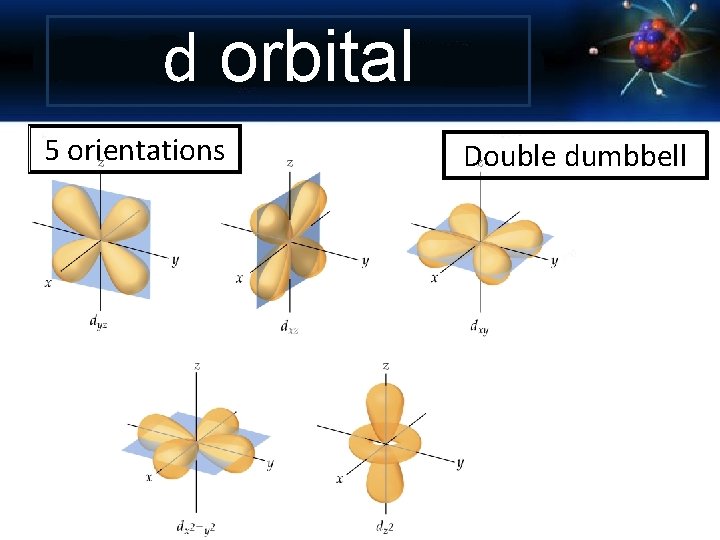
d orbital 5 orientations Double dumbbell

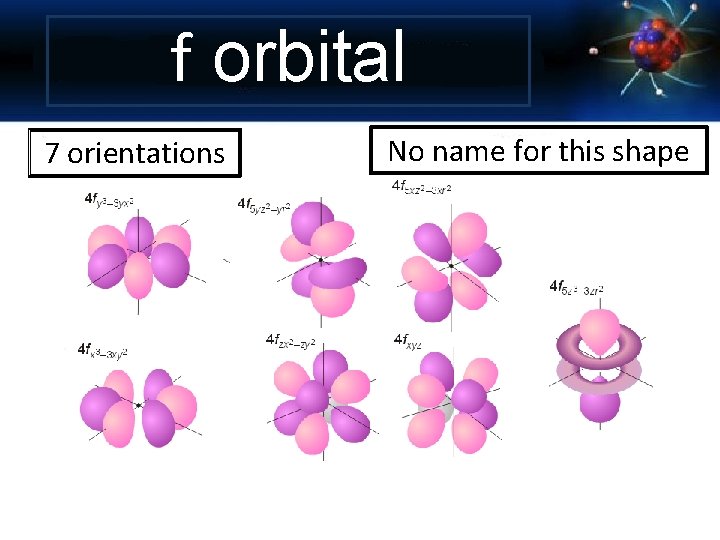
f orbital 7 orientations No name for this shape

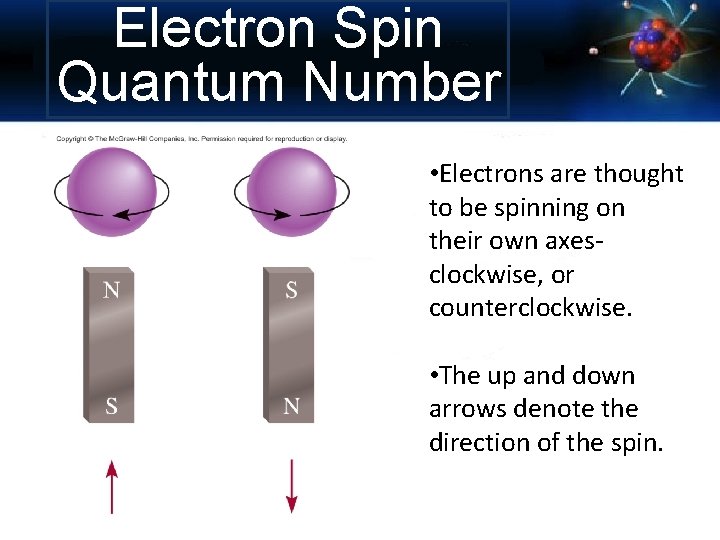
Electron Spin Quantum Number • Electrons are thought to be spinning on their own axesclockwise, or counterclockwise. • The up and down arrows denote the direction of the spin.

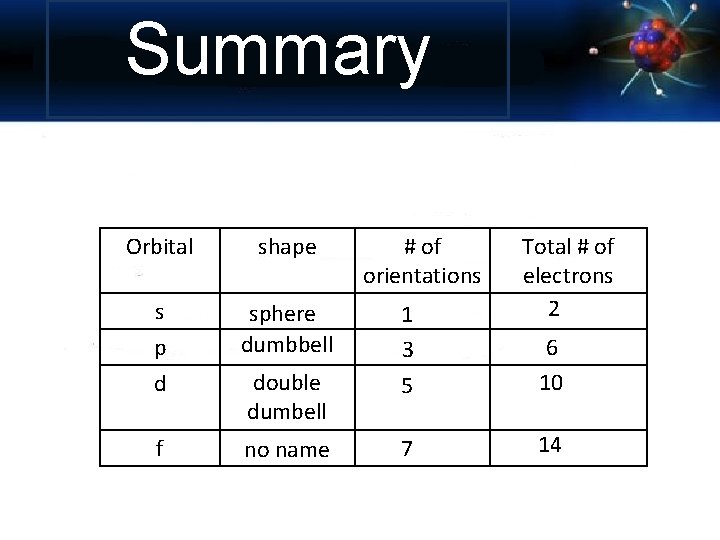
Summary Orbital shape # of orientations Total # of electrons 2 s p sphere dumbbell 1 3 d double dumbell 5 6 10 f no name 7 14


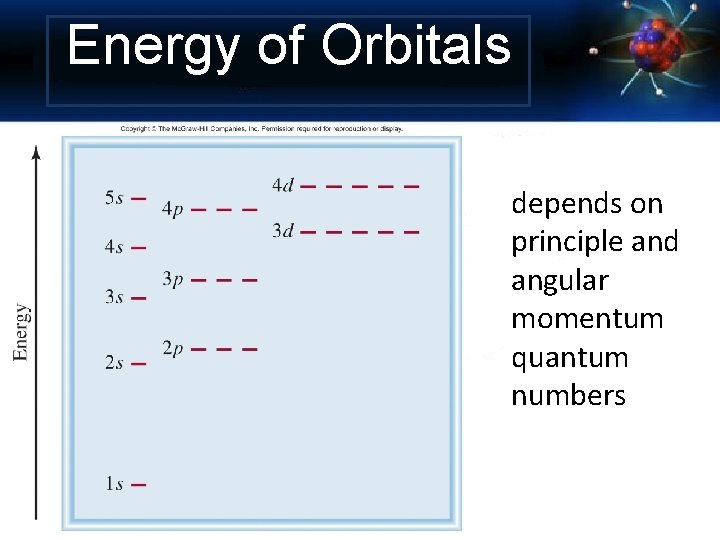
Energy of Orbitals depends on principle and angular momentum quantum numbers

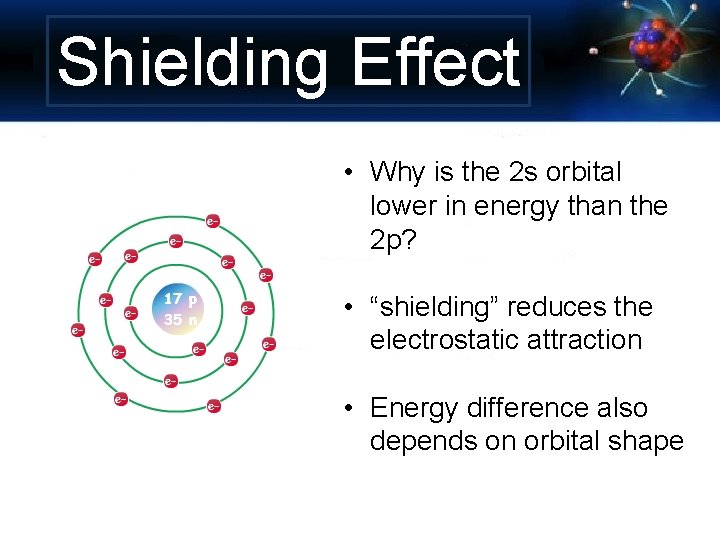
Shielding Effect • Why is the 2 s orbital lower in energy than the 2 p? • “shielding” reduces the electrostatic attraction • Energy difference also depends on orbital shape

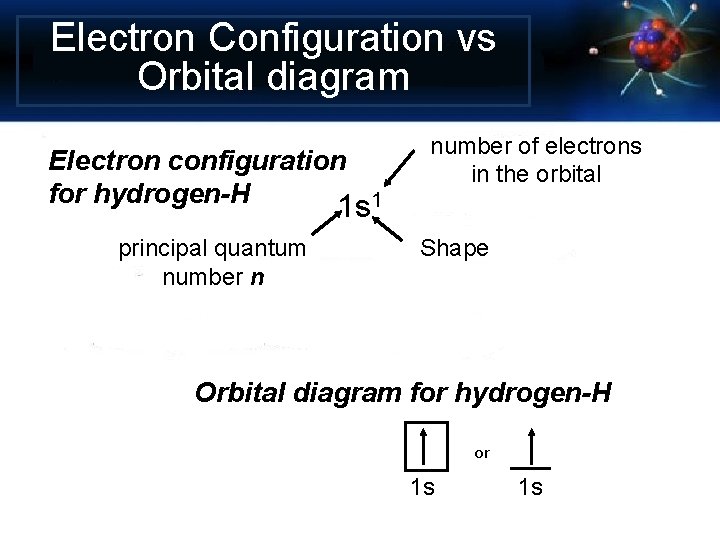
Electron Configuration vs Orbital diagram Electron configuration for hydrogen-H 1 s 1 principal quantum number n number of electrons in the orbital Shape Orbital diagram for hydrogen-H or 1 s 1 s

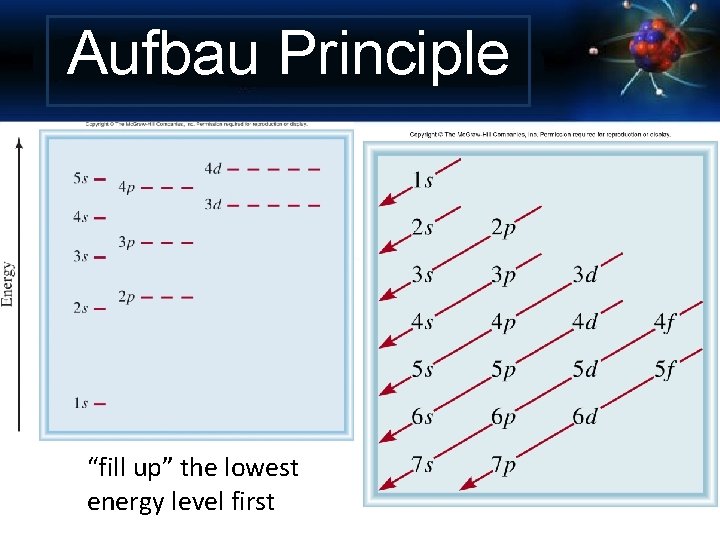
Aufbau Principle “fill up” the lowest energy level first

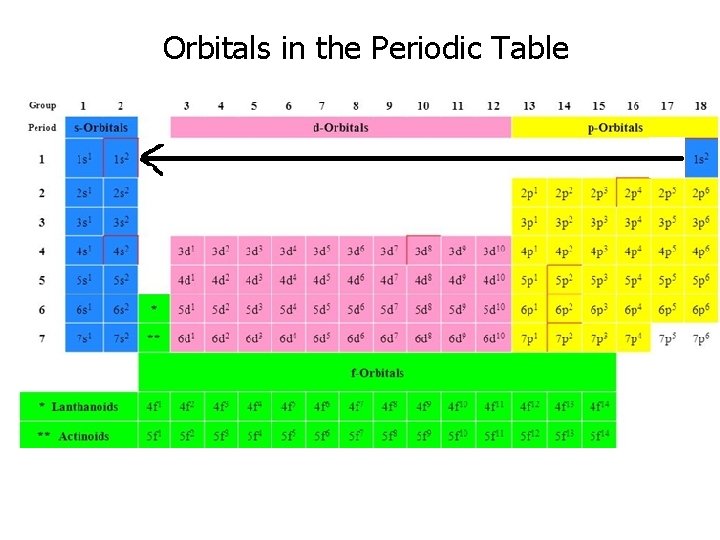
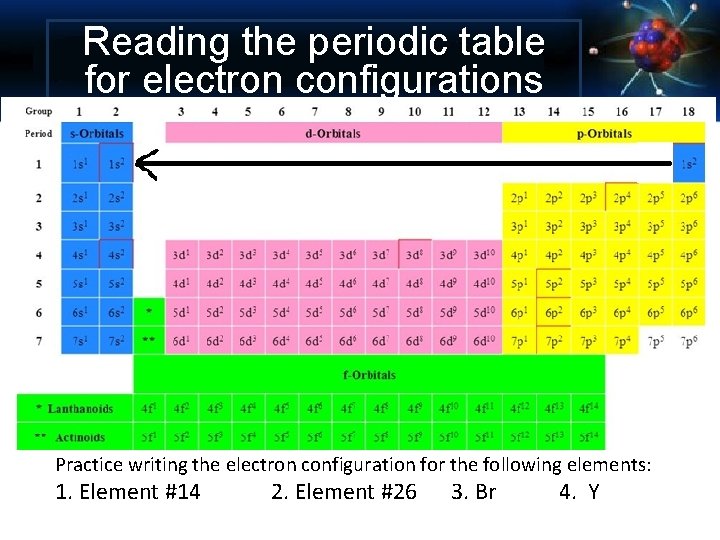
Orbitals in the Periodic Table

Pauli Exclusion Principle • No two electrons can have the same 4 quantum numbers • Only two electrons may occupy the same atomic orbital, and these electrons must have opposite spins • Electrons that have opposite spins are said to be paired

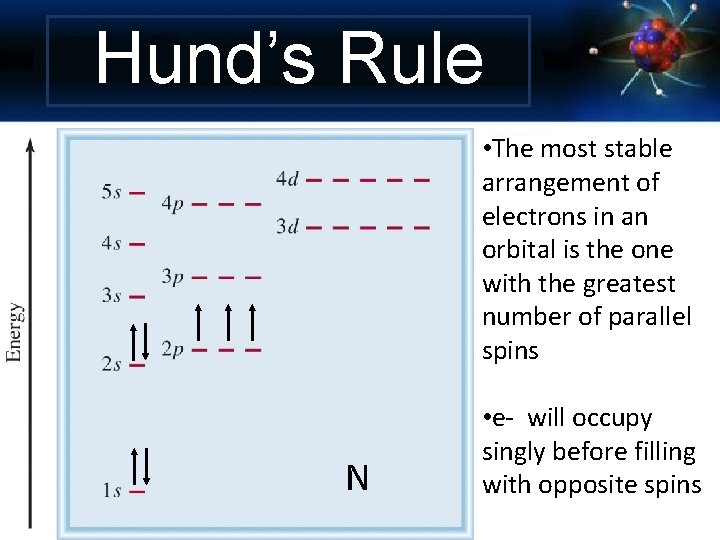
Hund’s Rule • The most stable arrangement of electrons in an orbital is the one with the greatest number of parallel spins N • e- will occupy singly before filling with opposite spins

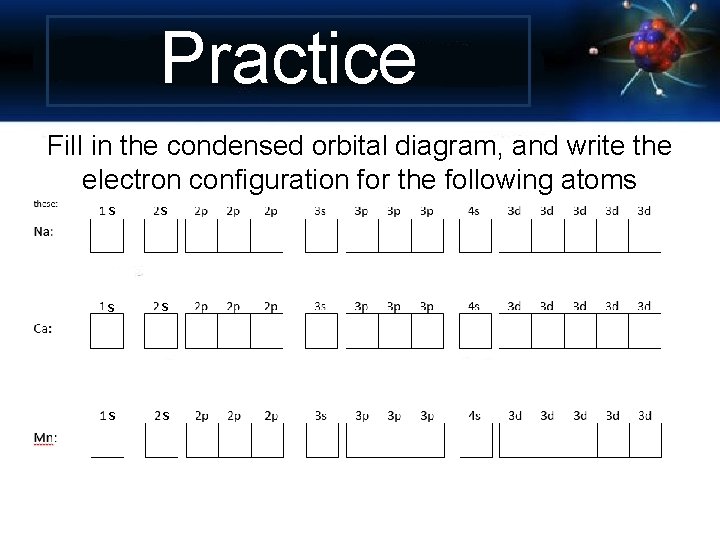
Practice Fill in the condensed orbital diagram, and write the electron configuration for the following atoms s s s

Day 2 -Exceptions to the rules and reading the table

Valence Electrons • Electrons in the outermost s and p orbitals (highest n shell) • These electrons participate in chemical reactions

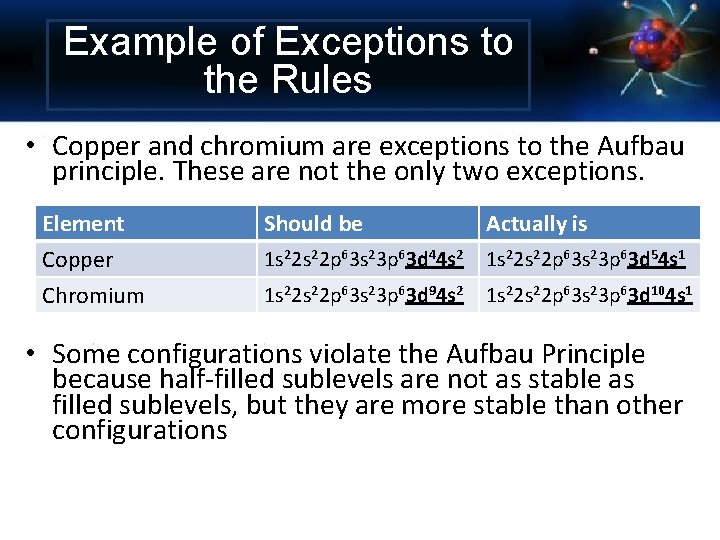
Example of Exceptions to the Rules • Copper and chromium are exceptions to the Aufbau principle. These are not the only two exceptions. Element Copper Chromium Should be Actually is 1 s 22 p 63 s 23 p 63 d 44 s 2 1 s 22 p 63 s 23 p 63 d 54 s 1 1 s 22 p 63 s 23 p 63 d 94 s 2 1 s 22 p 63 s 23 p 63 d 104 s 1 • Some configurations violate the Aufbau Principle because half-filled sublevels are not as stable as filled sublevels, but they are more stable than other configurations

Reading the periodic table for electron configurations Practice writing the electron configuration for the following elements: 1. Element #14 2. Element #26 3. Br 4. Y

Battleship fun WITH ELECTRON CONFIGURATIONS

Objective • How to play battleship using the periodic table • Set up periodic table s, p, d, f-block to resemble “battleship gameboard” • GOAL: Memorize the sequence while having fun. • Let’s play!

Prior to playing Students should be familiar with the concept of electron configuration Ideal if they know the sequence but not necessary Aufbau principle, Pauli Exclusion Principle, and Hund’s rule, and exceptions

The Real Deal • How is the “real” battleship game is played?

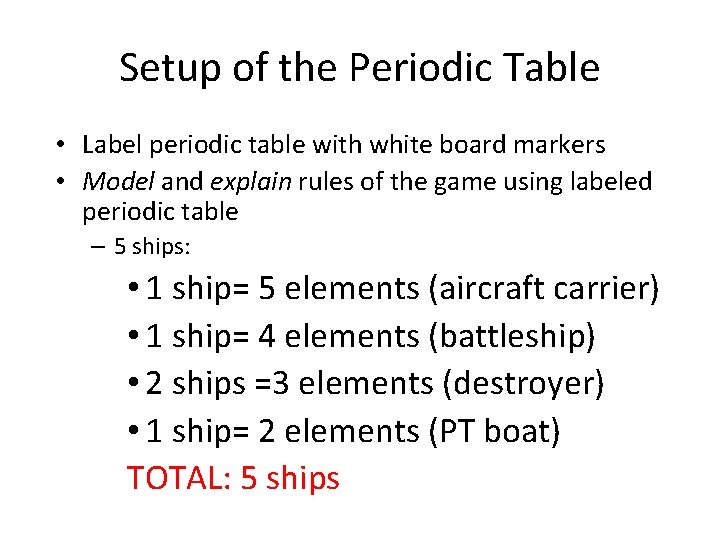
Setup of the Periodic Table • Label periodic table with white board markers • Model and explain rules of the game using labeled periodic table – 5 ships: • 1 ship= 5 elements (aircraft carrier) • 1 ship= 4 elements (battleship) • 2 ships =3 elements (destroyer) • 1 ship= 2 elements (PT boat) TOTAL: 5 ships

Playing the game • Use paper score card • Use bottom game board as the record for what is being hit • Use the top of the game board to record your ships • Two day game: Part 1 (electron config) and Part 2 (noble gas config) • Monitor student progress

After the game • Review how to READ the periodic table • complete PART 2 on the same sheet tomorrow • Revisit Aufbau principle, Pauli Exclusion Principle, and Hund’s rule, and exceptions

Noble Gas Configuration Day 3

Noble Gas Configuration What is the electron configuration for Ne? Ne: What is the electron configuration for Mg? Mg: What do both electron configurations have in common?

To figure out which noble gas to use find the noble gas that is closest to the element without going over in atomic number Which noble gas is closest without going over? Rb Cl Ra

Practice Write the noble gas electron configuration for the following atoms: Na: Mn: Co: Sn:

Heisenberg uncertainty principle (1927) states that it is impossible to know both the velocity and position of a particle at the same time.
