Phases of Matter and Phase Changes Phase l






- Slides: 20

Phases of Matter and Phase Changes

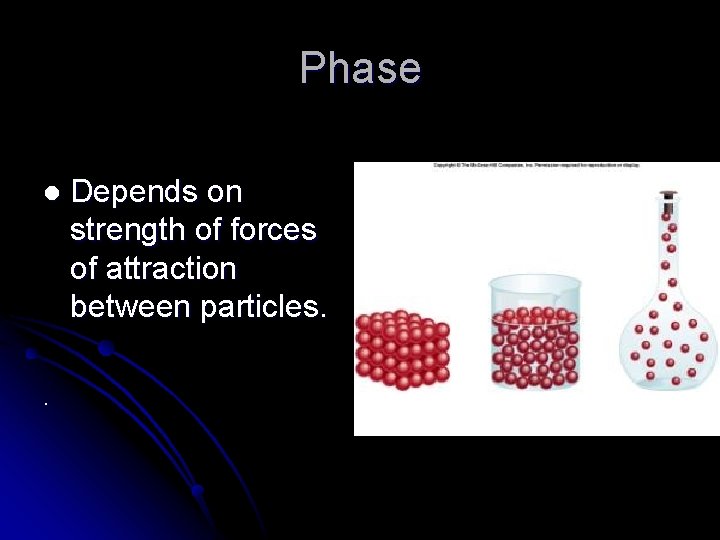
Phase l . Depends on strength of forces of attraction between particles.

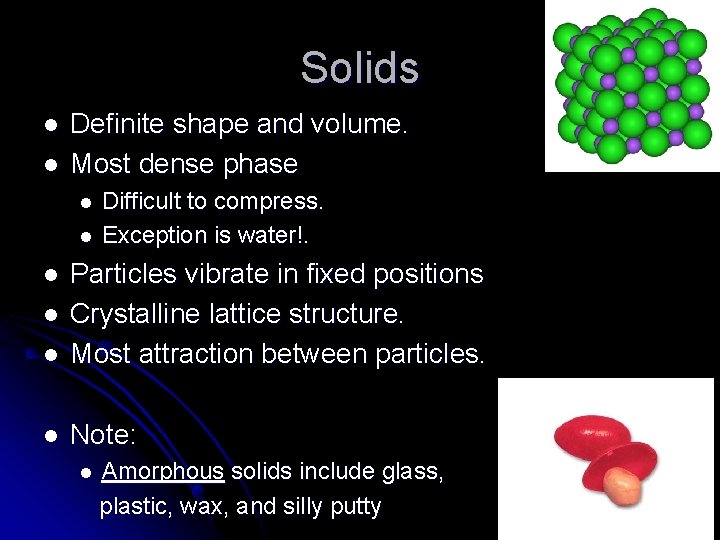
Solids l l Definite shape and volume. Most dense phase l l Difficult to compress. Exception is water!. l Particles vibrate in fixed positions Crystalline lattice structure. Most attraction between particles. l Note: l l l Amorphous solids include glass, plastic, wax, and silly putty

Liquids Definite volume l No definite shape l Hard to compress l Particles slide past each other l Forces of attraction between particles still high l

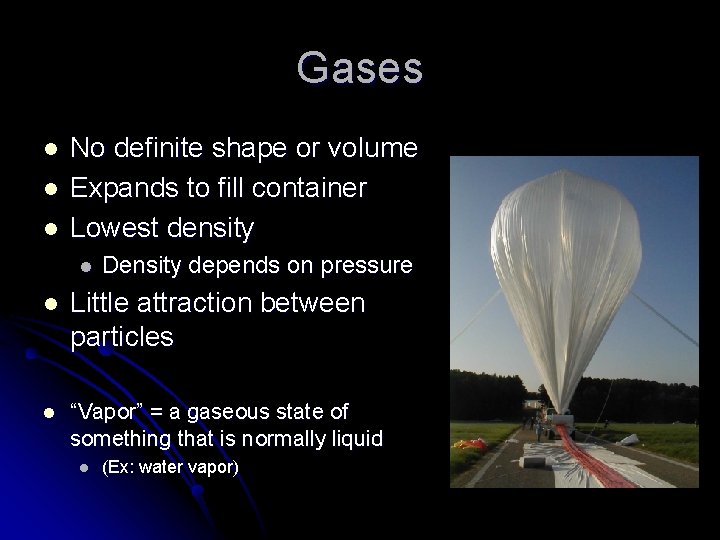
Gases l l l No definite shape or volume Expands to fill container Lowest density l Density depends on pressure l Little attraction between particles l “Vapor” = a gaseous state of something that is normally liquid l (Ex: water vapor)

Phases Applet l Short Summary video on phases: (1 min) l http: //www. youtube. com/watch? v=s- Kvo. Vzuk. Ho&safe=active l Applet: (Excellent) l https: //phet. colorado. edu/en/simulation/states- of-matter l http: //www. harcourtschool. com/activity/states_ of_matter/

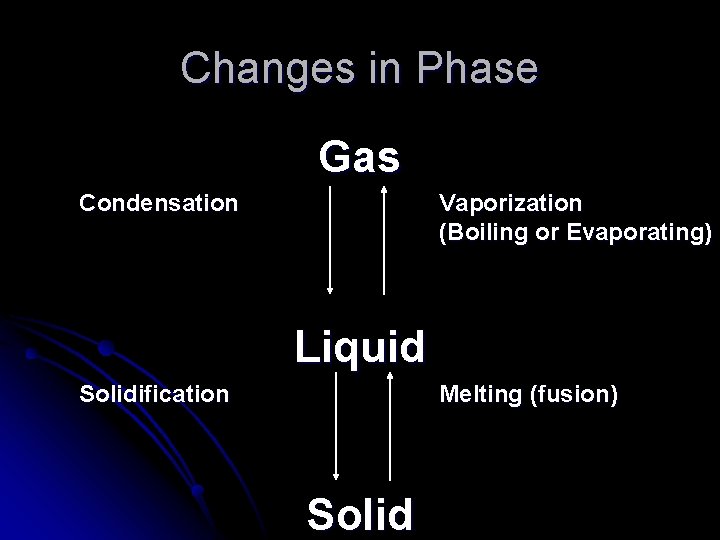
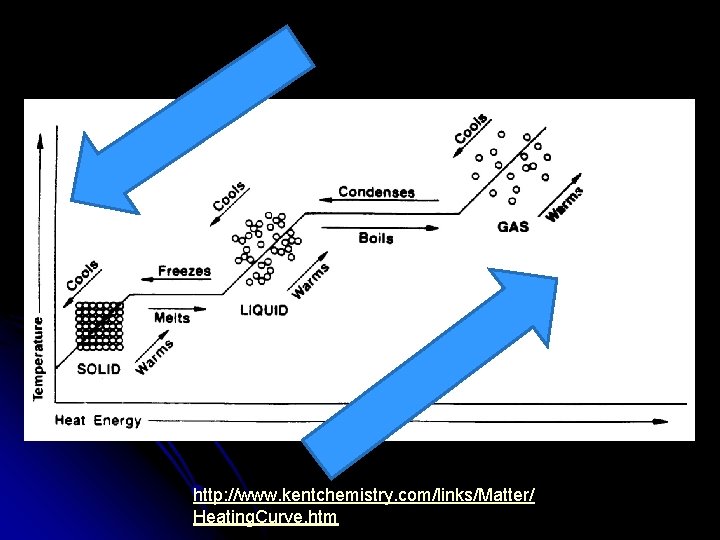
Changes in Phase Gas Condensation Vaporization (Boiling or Evaporating) Liquid Solidification Melting (fusion) Solid

Let’s Skip a Phase l Sublimation l Directly from the solid phase to the gas phase. l Happens with substances with weak intermolecular forces of attraction l They separate easily! l Ex: CO 2(s) dry ice, Iodine CO 2(s) → CO 2 (g) http: //www. youtube. com/watch? v=8 t. HOVVg. Gkpk

Energy l Energy = capacity to do work or produce heat. It can be anything that causes matter to move or change direction. l Ex: electrical, atomic, mechanical, chemical

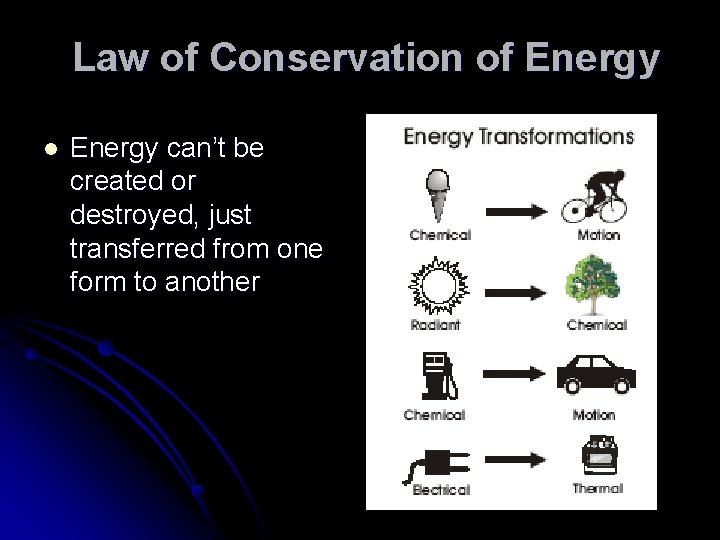
Law of Conservation of Energy l Energy can’t be created or destroyed, just transferred from one form to another

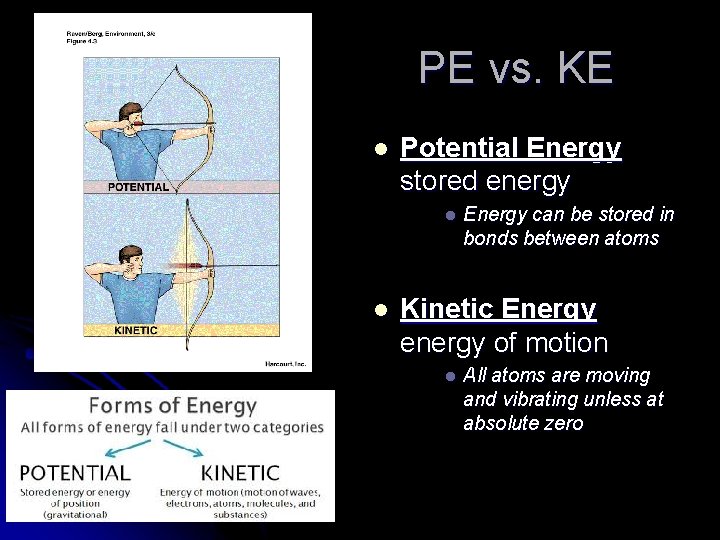
PE vs. KE l Potential Energy stored energy l l Energy can be stored in bonds between atoms Kinetic Energy energy of motion l All atoms are moving and vibrating unless at absolute zero

Energy and Changes to Matter l Exothermic Change: l l A + B → C + D + energy Energy is released or “ex”its Endothermic Change: A + B + energy → C + D l Energy is absorbed or “en”ters

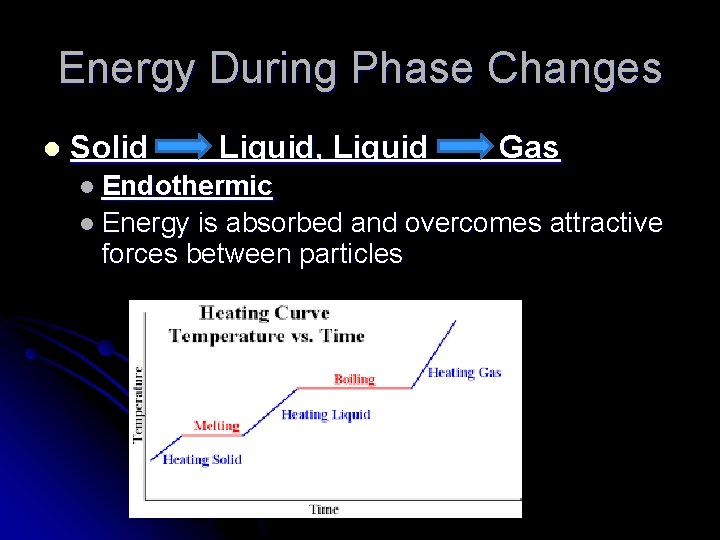
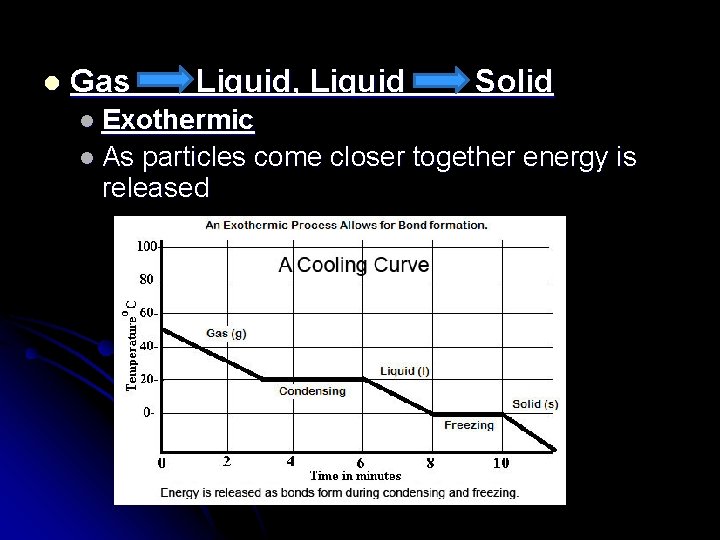
Energy During Phase Changes l Solid Liquid, Liquid Gas l Endothermic l Energy is absorbed and overcomes attractive forces between particles

l Gas Liquid, Liquid Solid l Exothermic l As particles come closer together energy is released

http: //www. kentchemistry. com/links/Matter/ Heating. Curve. htm

Heat Energy l Also called Thermal energy, it makes particles move more as it is added l Measured in Joules or calories. http: //www. youtube. com/watch? v=f 1 e. AOyg. DP 5 s&safe=active

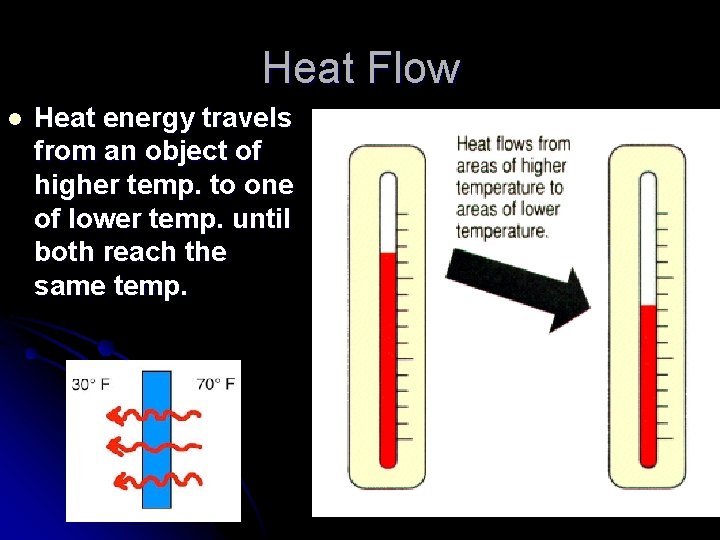
Heat Flow l Heat energy travels from an object of higher temp. to one of lower temp. until both reach the same temp.

Temperature l Measure of the average kinetic energy (motion) of all the particles in a sample. l Not a form of energy!!! l But if you add heat energy or take it away, it causes particles to move faster or slower and thus changes the temp.

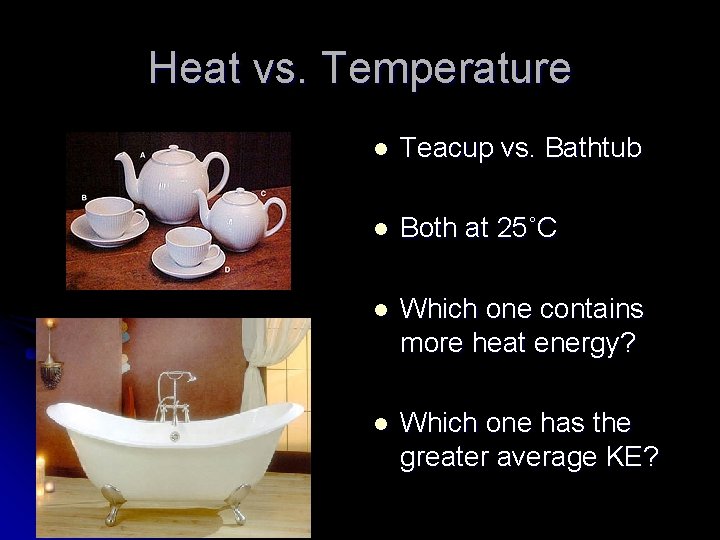
Heat vs. Temperature l Teacup vs. Bathtub l Both at 25˚C l Which one contains more heat energy? l Which one has the greater average KE?

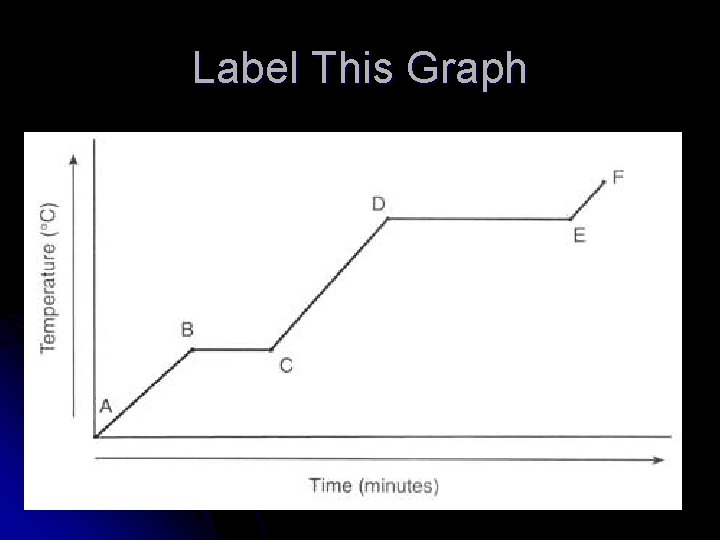
Label This Graph