States Phases of Matter and Phase Changes Unit











- Slides: 25

States (Phases) of Matter and Phase Changes Unit 5: Phases and Phase Changes of Matter

Do-Now (1/29): 1. How come you can stand on a glacier but not on the ocean water that is surrounding the glacier? 2. Does a pure substance always have the same density value? Explain your answer.

e l c i t r a P ture a N f o r e t t a M All Matter is made up of tiny particles, and it is important to remember the following characteristics when describing a phase or phase change: I. The particles of matter have space in between them II. The particles of matter are continuously moving III. Adding heat makes the particles move faster, and/or breaks the attractions between the particles.

s e t a St atter M f o PURE substances are commonly found in one of 3 different states, or phases: solids, liquids and gases. Less commonly, they can exist in a state known as a plasma

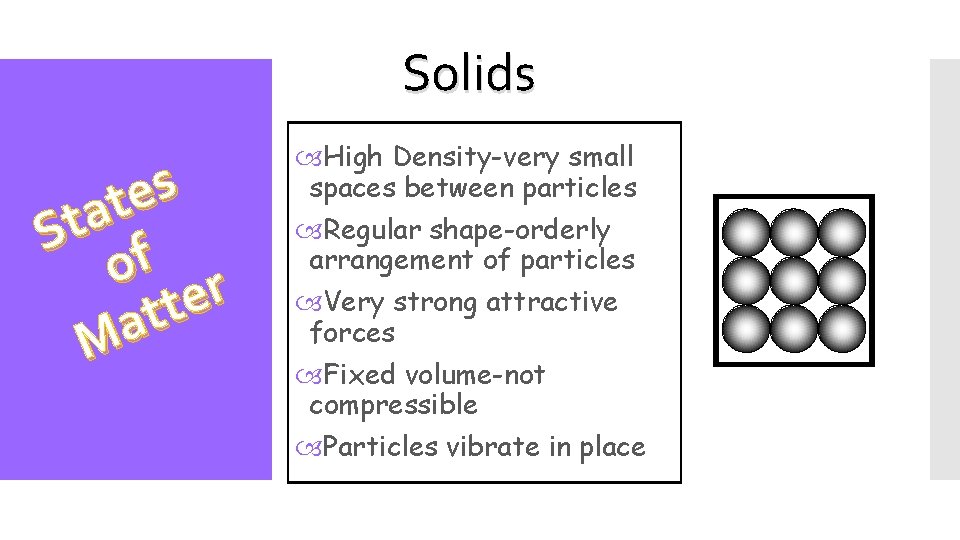
Solids s e t a St f o r e t t a M High Density-very small spaces between particles Regular shape-orderly arrangement of particles Very strong attractive forces Fixed volume-not compressible Particles vibrate in place

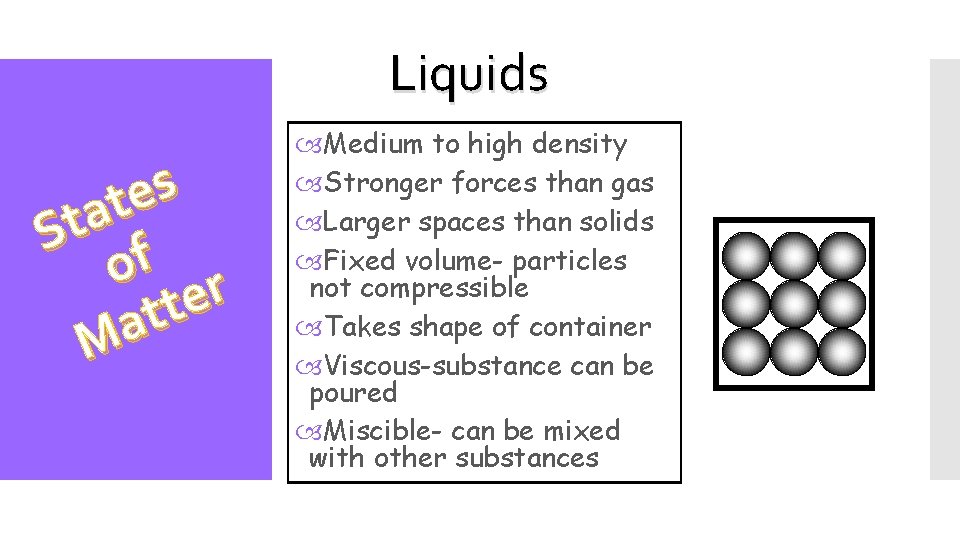
Liquids s e t a St f o r e t t a M Medium to high density Stronger forces than gas Larger spaces than solids Fixed volume- particles not compressible Takes shape of container Viscous-substance can be poured Miscible- can be mixed with other substances

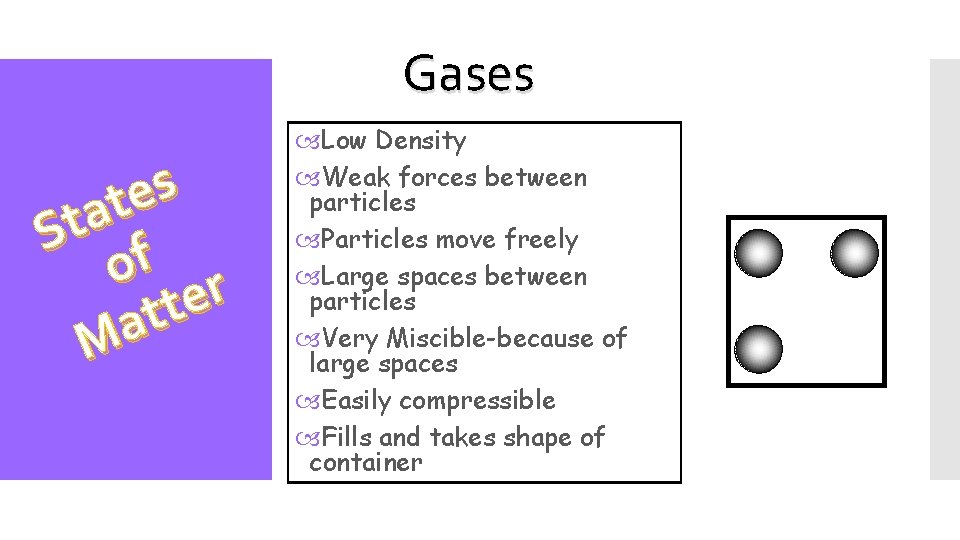
Gases s e t a St f o r e t t a M Low Density Weak forces between particles Particles move freely Large spaces between particles Very Miscible-because of large spaces Easily compressible Fills and takes shape of container

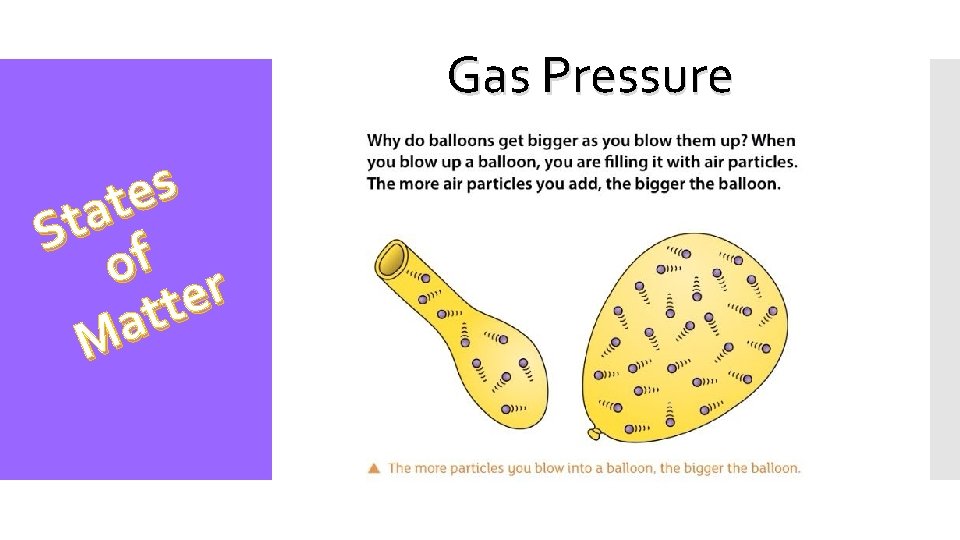
Gas Pressure s e t a St f o r e t t a M Gases exert pressure on any surface they come in contact with. Pressure is related to the number of collisions the gas molecules have with wall of a container. More collisions = More pressure Pressure = Force / Area The force of impact of a single collision is too small to be sensed. Taken all together, this large number of impacts of gas molecules exerts a large force on a surface.

Gas Pressure s e t a St f o r e t t a M

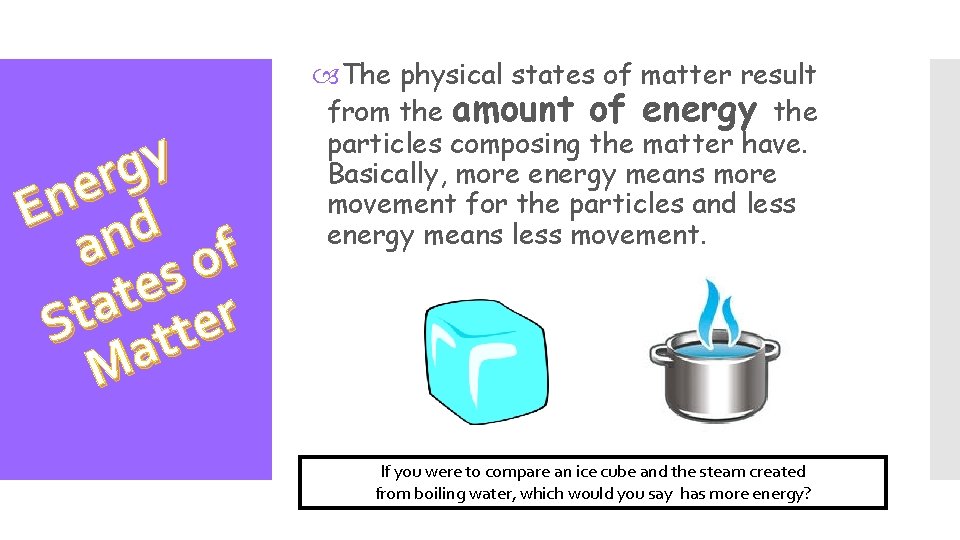
y g r e En d an of s e t a St atter M The physical states of matter result from the amount of energy the particles composing the matter have. Basically, more energy means more movement for the particles and less energy means less movement. If you were to compare an ice cube and the steam created from boiling water, which would you say has more energy?

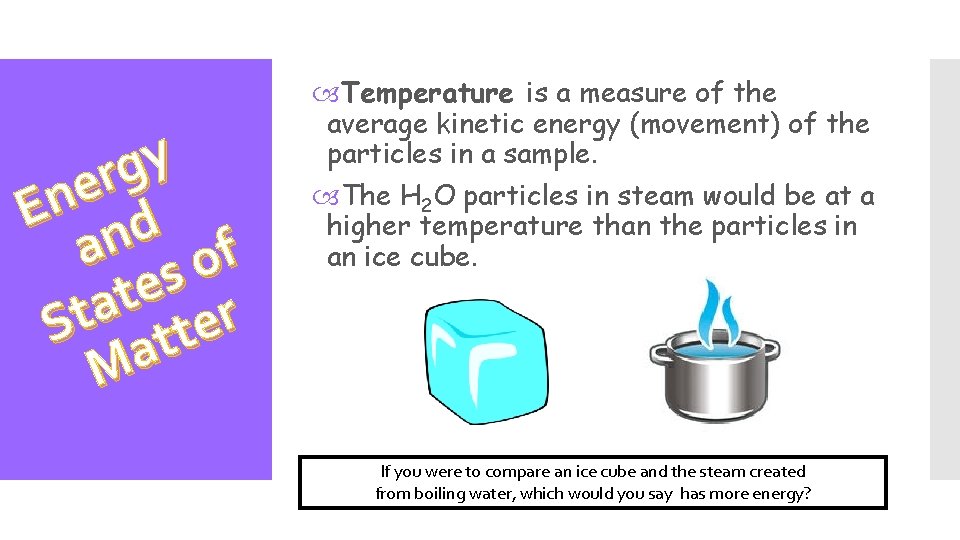
y g r e En d an of s e t a St atter M Temperature is a measure of the average kinetic energy (movement) of the particles in a sample. The H 2 O particles in steam would be at a higher temperature than the particles in an ice cube. If you were to compare an ice cube and the steam created from boiling water, which would you say has more energy?

y g r e En d an of s e t a St atter M The Melting Point of a substance is the temperature at which the particles shift from the solid phase to the liquid phase. The Boiling Point of a substance is the temperature at which the particles shift from the liquid phase to the gas phase. The melting and boiling points of a substance will determine what phase a substance is in at room temperature.

y g r e En d an of s e t a St atter M Phase Changes result from the particles of matter either gaining or losing a certain amount energy Endothermic-energy (heat) is absorbed by the system Exothermic- energy (heat) is released by the system Phase Changes are Reversible Physical Changes!

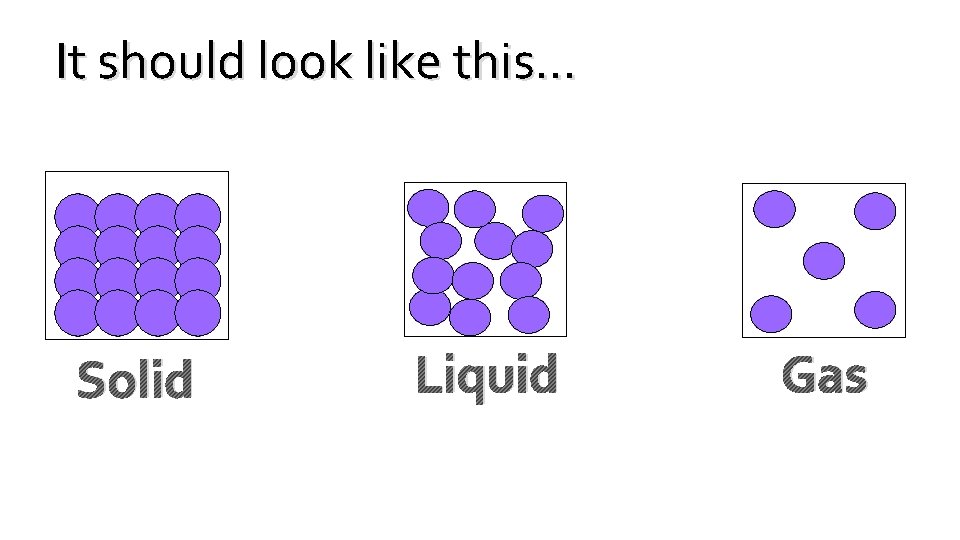
• Draw Three (3) Boxes equally spaced across your paper like so: • Next, label “Solid” underneath the Left Box, the Middle Box "Liquid", and the Right Box "Gas" • Lastly, draw what the particles would look like inside each of the three different States of Matter.

It should look like this… Solid Liquid Gas

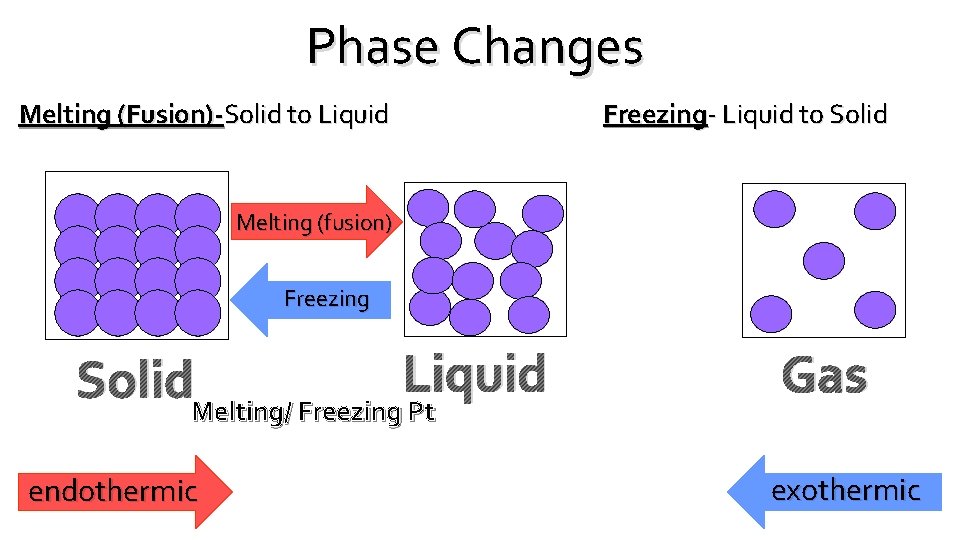
Phase Changes Melting (Fusion)-Solid to Liquid Freezing- Liquid to Solid Melting (fusion) Freezing Solid. Melting/ Freezing Liquid Pt endothermic Gas exothermic

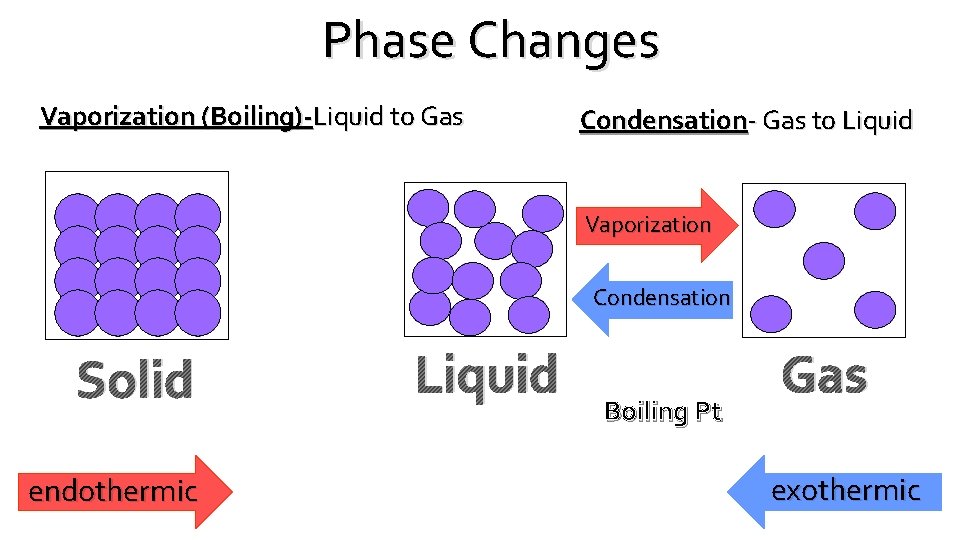
Phase Changes Vaporization (Boiling)-Liquid to Gas Condensation- Gas to Liquid Vaporization Condensation Solid endothermic Liquid Boiling Pt Gas exothermic

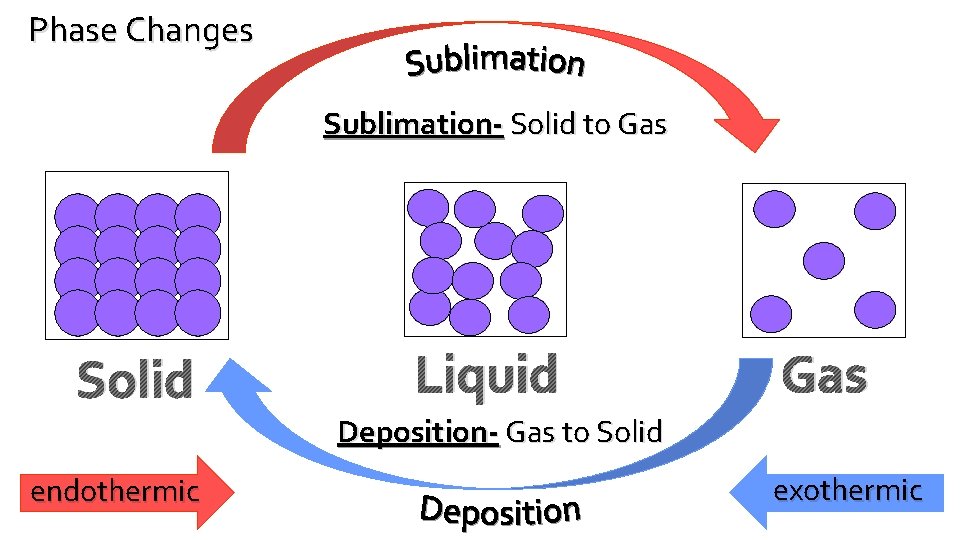
Phase Changes Sublimation- Solid to Gas Solid endothermic Liquid Gas Deposition- Gas to Solid exothermic

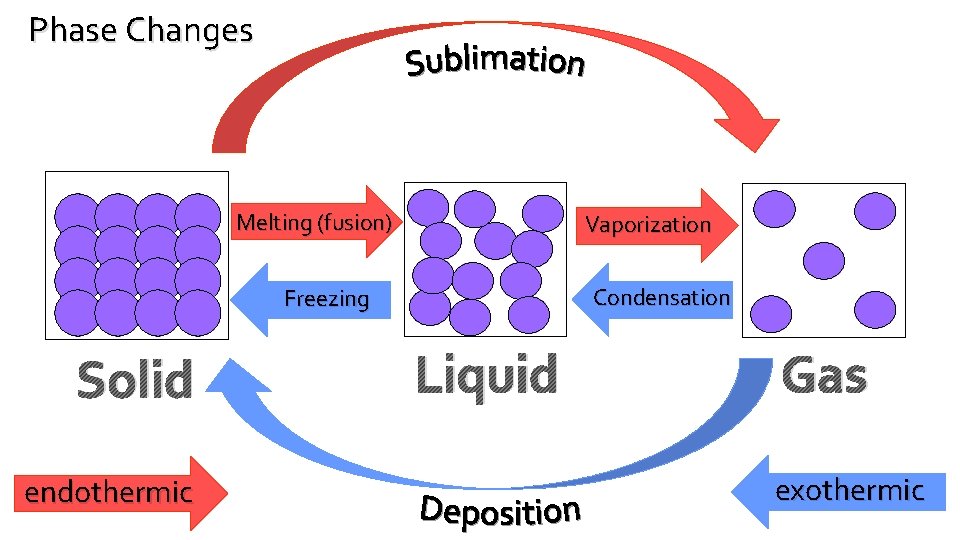
Phase Changes Melting (fusion) Vaporization Condensation Freezing Solid endothermic Liquid Gas exothermic

e c i t c s a r n P stio e u Q 1. Which of the following is a property that is shared with all particles of matter? a. Never move in solids. b. Only move in gases. c. Move constantly. d. They move in liquid and gases only.

e c i t c s a r n P stio e u Q 2. If I am adding energy into a solid and it becomes a liquid, then this is an example of ______ which is an ______ type of change. a. Melting; exothermic b. Melting; endothermic c. Freezing; exothermic d. Freezing; endothermic

e c i t c s a r n P stio e u Q 3. On a hot day, you notice that your drink can starts to form beads of water on its sides. This is a sign that _____ starts to occur, which is an example of an _______ change. a. Evaporation; exothermic b. Evaporation; endothermic c. Condensation; exothermic d. Condensation; endothermic

e c i t c s a r n P stio e u Q 4. Use the two boxes below to detail what is occurring to the particles of H 2 O during the scenario discussed in question 3. Be as descriptive as possible.

5. Which of the following statements best describes the particles of a liquid? e c i t c s a r n P stio e u Q a. The particles are far apart and moving fast. b. The particles are close together but moving past each other. c. The particles are far apart and moving slowly. d. The particles are closely packed and vibrating in place.

e c i t c s a r n P stio e u Q 6. Explain why energy is needed to change a state.