Organic Chemistry Chapter 12 Oxygen and Sulfur in



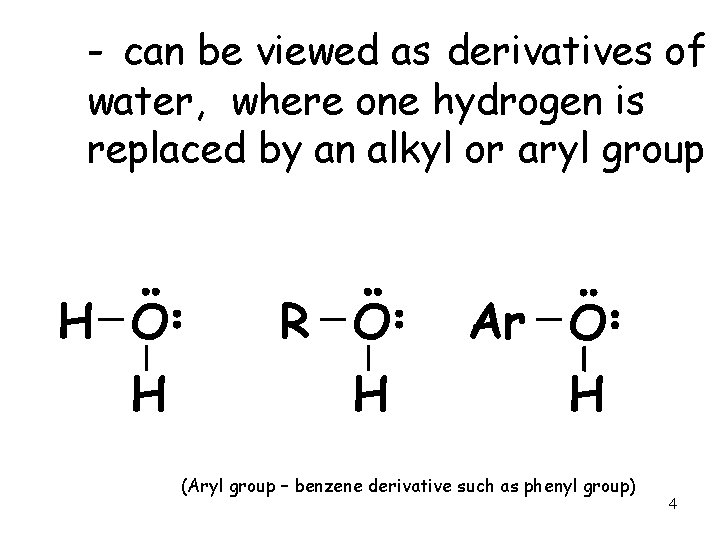



- Slides: 23

Organic Chemistry Chapter 12 Oxygen and Sulfur in Organic Compounds 1

Part 01 The Alcohols 2

Alcohols • Organic molecules that contain the hydroxyl functional group, OH • General formula for an alcohol is ROH 3
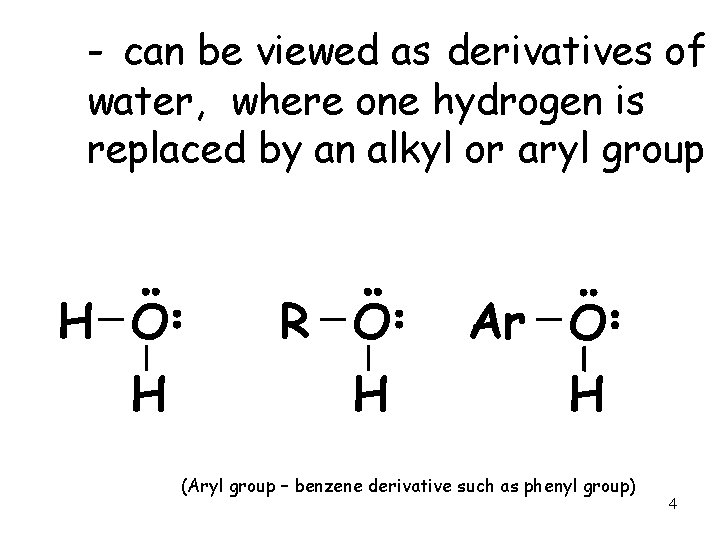
- can be viewed as derivatives of water, where one hydrogen is replaced by an alkyl or aryl group H O H R O H Ar O H (Aryl group – benzene derivative such as phenyl group) 4

Classification of Alcohols • Primary alcohol, 1º • Secondary alcohol, 2º • Tertiary alcohol, 3º 5

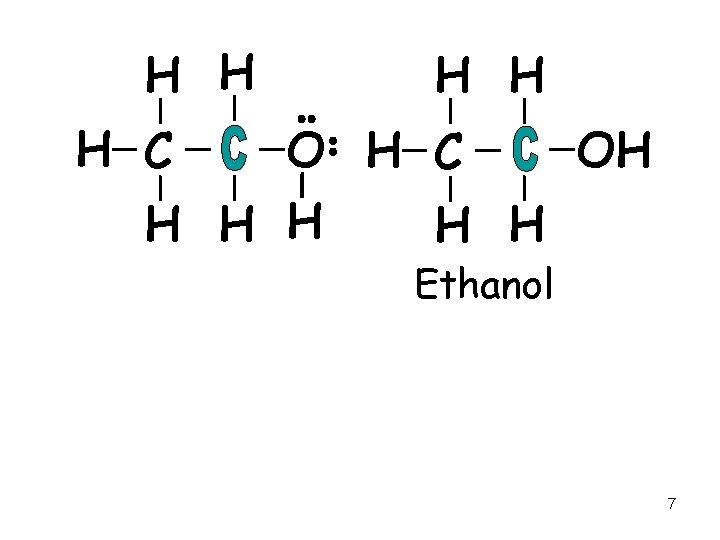
Classification of Alcohols • Primary alcohol, 1º - has one R group and 2 Hs on the carbinol carbon - C to which the –OH is bonded 6

H H H C OH H H Ethanol 7

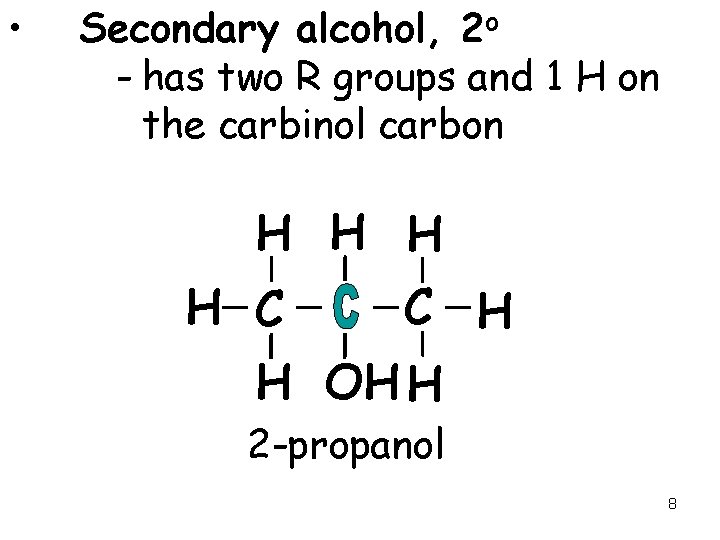
• Secondary alcohol, 2 o - has two R groups and 1 H on the carbinol carbon H H C C H H OH H 2 -propanol 8

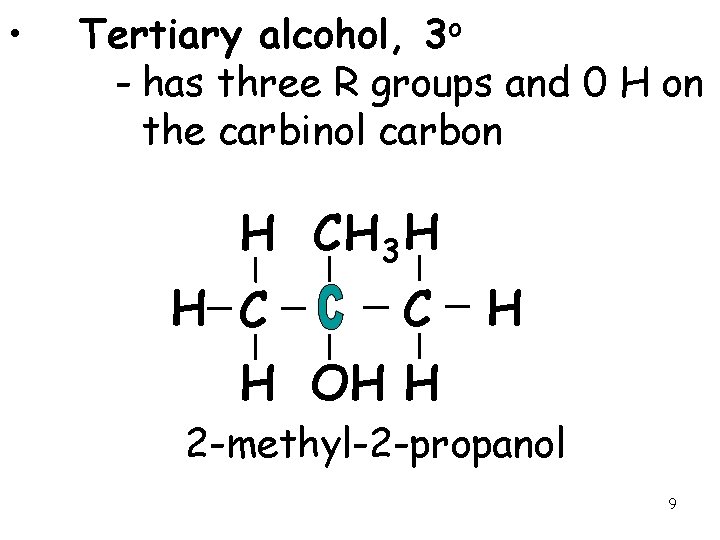
• Tertiary alcohol, 3 o - has three R groups and 0 H on the carbinol carbon H CH 3 H H C C H H OH H 2 -methyl-2 -propanol 9

Nomenclature of alcohols • • All names end in -ol Determine parent chain (longest continuous chain that contains the – OH group) The alcohol group has priority in numbering the chain If more than 1 hydroxyl group, use di or tri before the ol • diols or triols • diols are known as glycols • Keep the entire parent chain name; don’t chop 10 off any letters. (i. e. pentanediol)

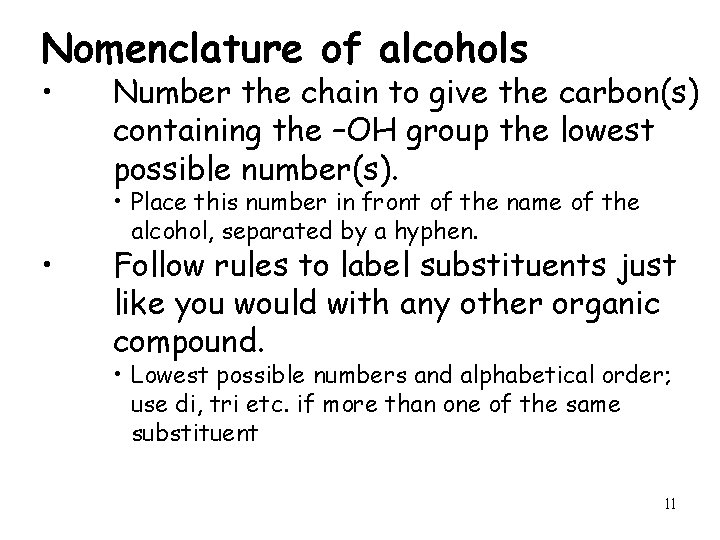
Nomenclature of alcohols • • Number the chain to give the carbon(s) containing the –OH group the lowest possible number(s). • Place this number in front of the name of the alcohol, separated by a hyphen. Follow rules to label substituents just like you would with any other organic compound. • Lowest possible numbers and alphabetical order; use di, tri etc. if more than one of the same substituent 11

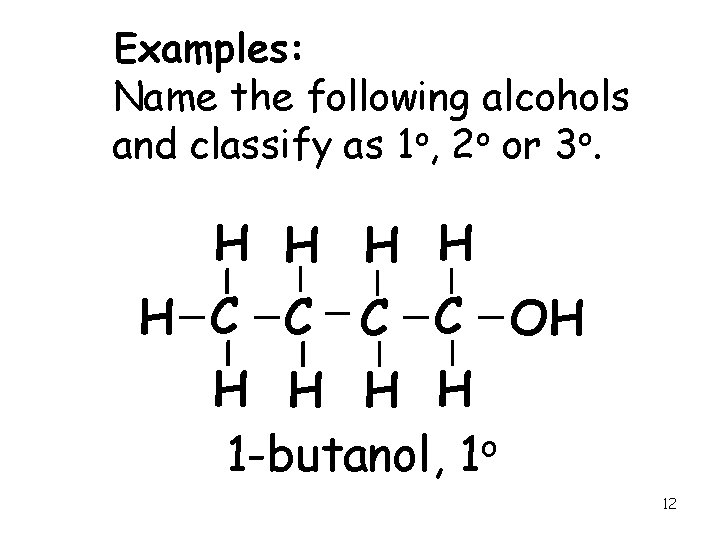
Examples: Name the following alcohols and classify as 1 o, 2 o or 3 o. H H H C C OH H H 1 -butanol, 1 o 12

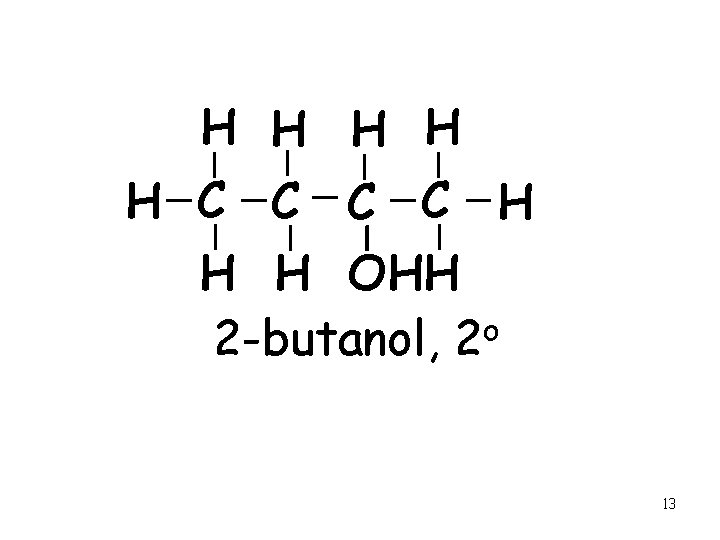
H H H C C H H H OHH 2 -butanol, 2 o 13

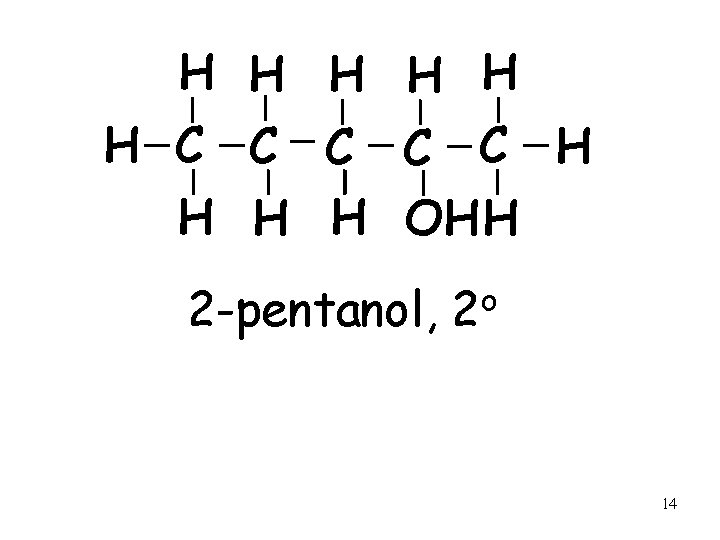
H H H C C C H H OH H 2 -pentanol, o 2 14

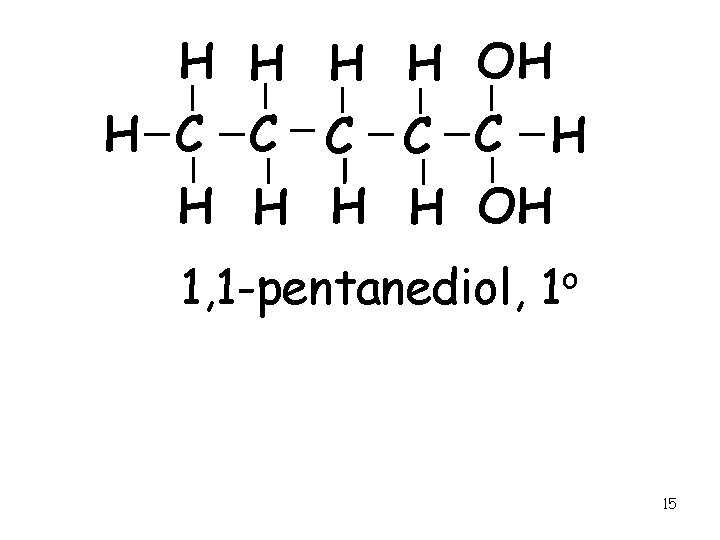
H H OH H C C C H H H OH 1, 1 -pentanediol, 1 o 15

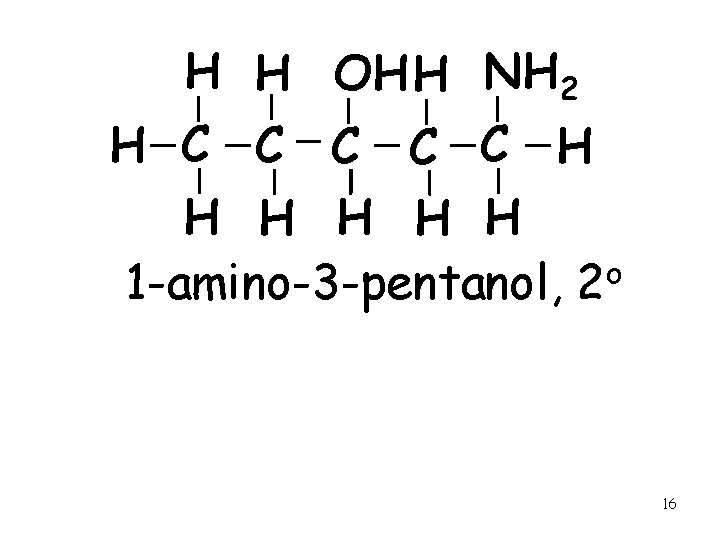
H H OH H NH 2 H C C C H H H 1 -amino-3 -pentanol, 2 o 16

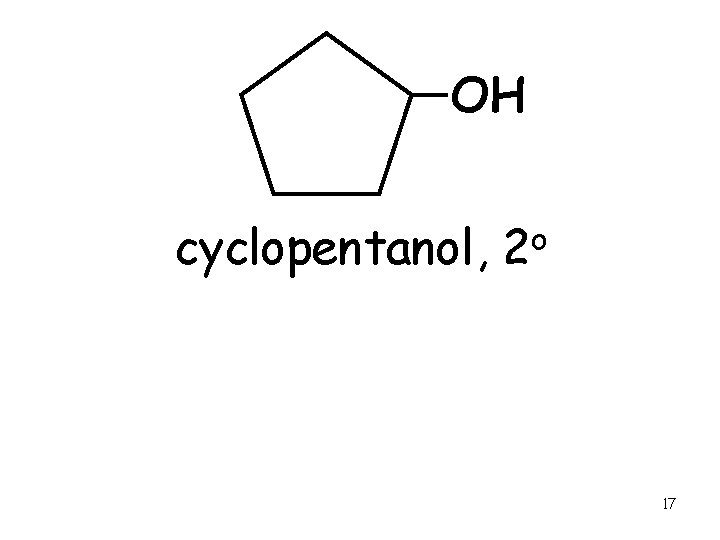
OH cyclopentanol, o 2 17

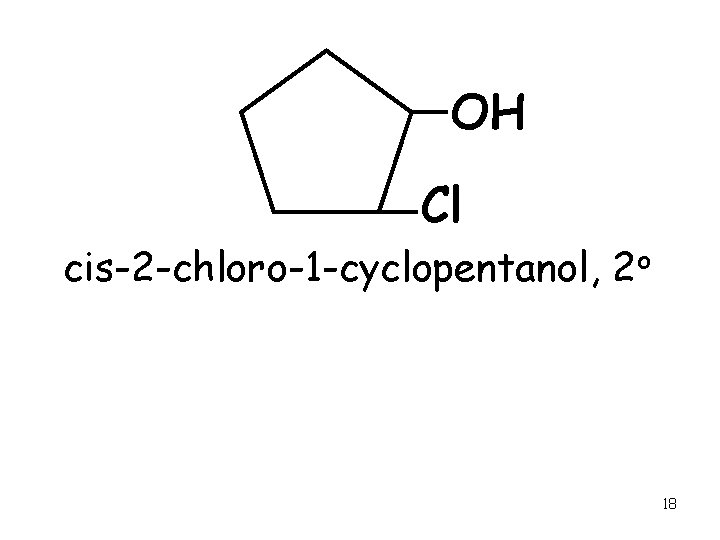
OH Cl cis-2 -chloro-1 -cyclopentanol, 2 o 18

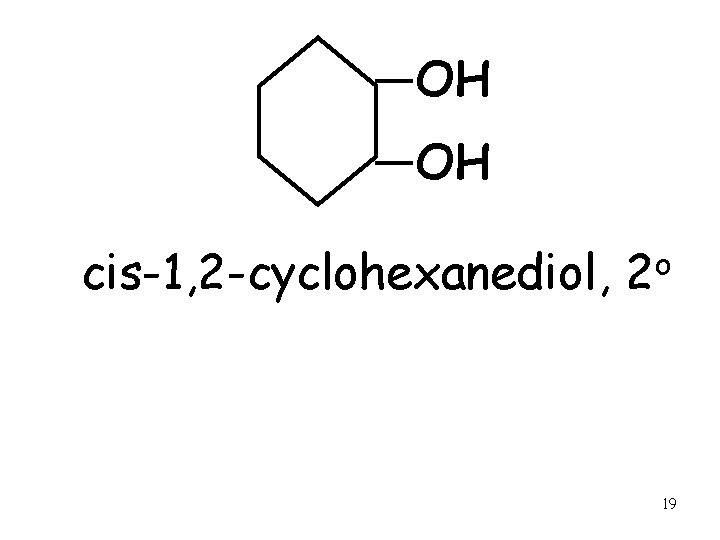
OH OH cis-1, 2 -cyclohexanediol, o 2 19

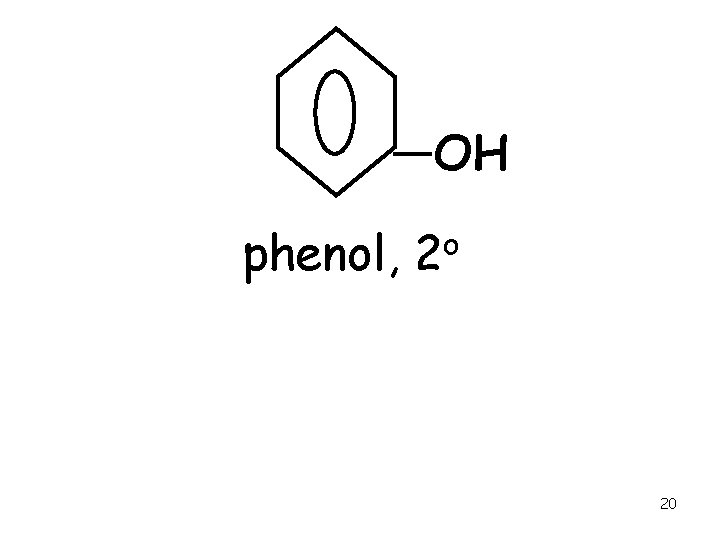
OH phenol, o 2 20

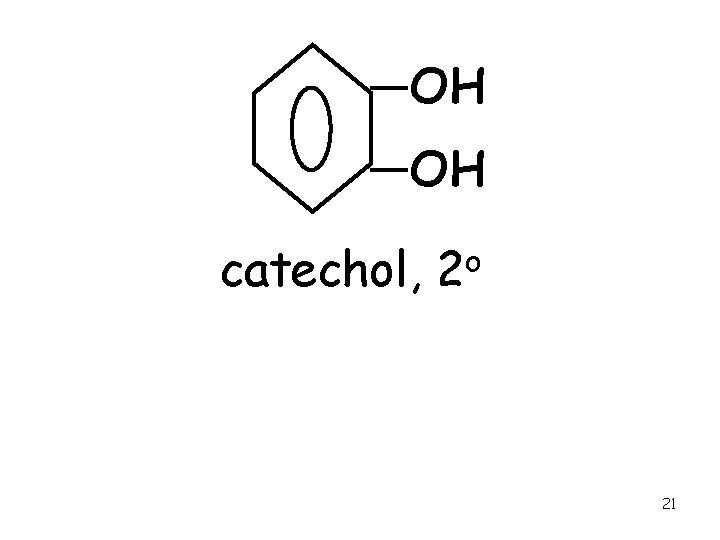
OH OH catechol, o 2 21

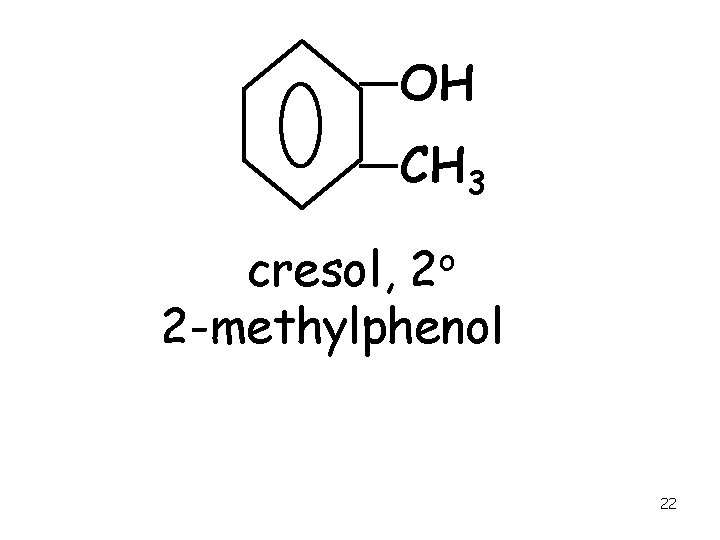
OH CH 3 o 2 cresol, 2 -methylphenol 22

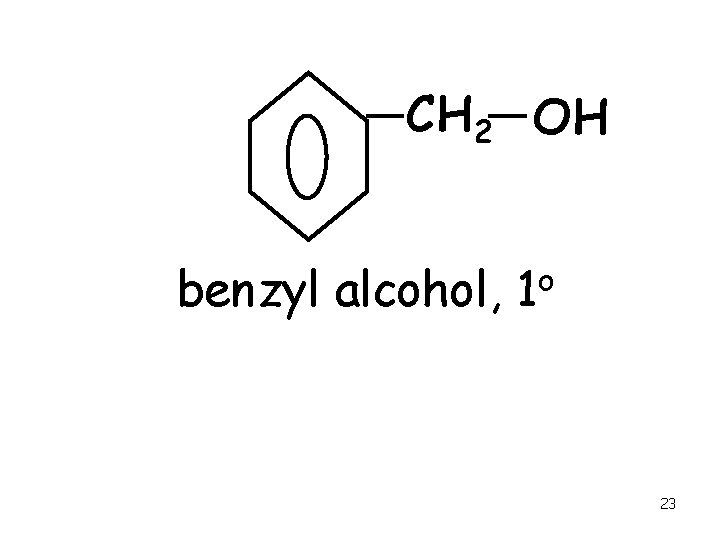
CH 2 OH benzyl alcohol, o 1 23
 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Importance of organic compounds
Importance of organic compounds Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Nonene
Nonene Analytical chemistry chapters
Analytical chemistry chapters Halohydrin formation
Halohydrin formation Define compound lipids
Define compound lipids Lewis dot structure ch4
Lewis dot structure ch4 Organic synthesis via enolates
Organic synthesis via enolates Cycloalkanes
Cycloalkanes Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Examples of isomers in chemistry
Examples of isomers in chemistry Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Nomenclature of ethers
Nomenclature of ethers Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein What is the leveling effect organic chemistry
What is the leveling effect organic chemistry