Formula Equations 1 Sulfur Oxygen Sulfur dioxide S





- Slides: 30

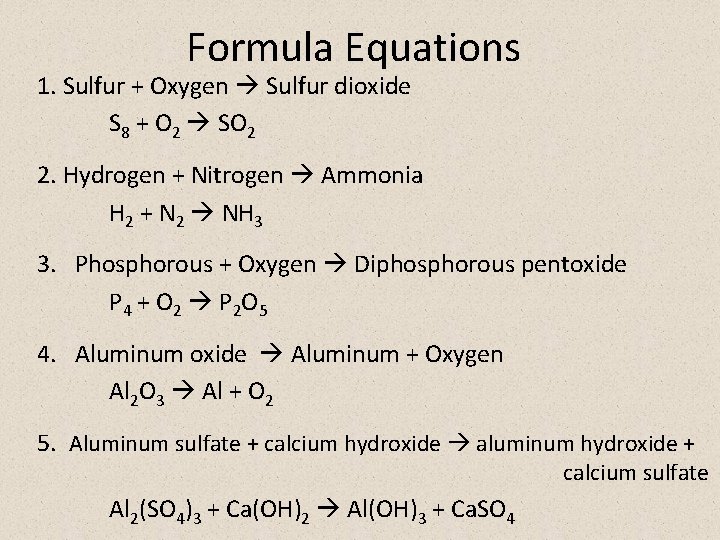
Formula Equations 1. Sulfur + Oxygen Sulfur dioxide S 8 + O 2 SO 2 2. Hydrogen + Nitrogen Ammonia H 2 + N 2 NH 3 3. Phosphorous + Oxygen Diphosphorous pentoxide P 4 + O 2 P 2 O 5 4. Aluminum oxide Aluminum + Oxygen Al 2 O 3 Al + O 2 5. Aluminum sulfate + calcium hydroxide aluminum hydroxide + calcium sulfate Al 2(SO 4)3 + Ca(OH)2 Al(OH)3 + Ca. SO 4

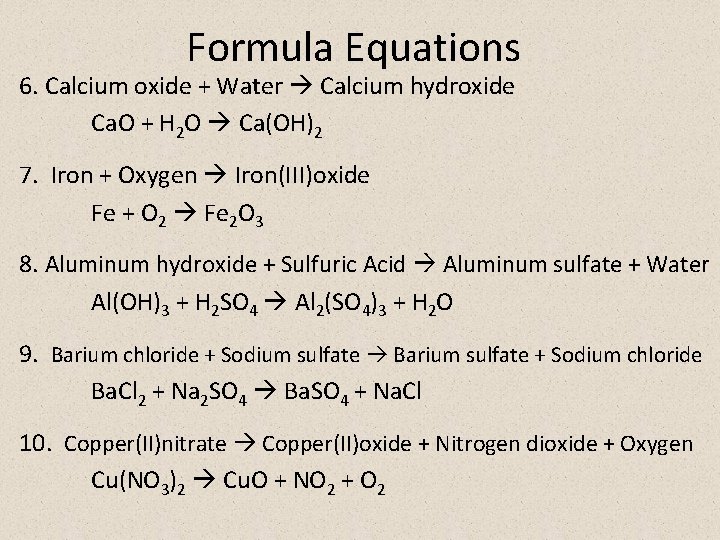
Formula Equations 6. Calcium oxide + Water Calcium hydroxide Ca. O + H 2 O Ca(OH)2 7. Iron + Oxygen Iron(III)oxide Fe + O 2 Fe 2 O 3 8. Aluminum hydroxide + Sulfuric Acid Aluminum sulfate + Water Al(OH)3 + H 2 SO 4 Al 2(SO 4)3 + H 2 O 9. Barium chloride + Sodium sulfate Barium sulfate + Sodium chloride Ba. Cl 2 + Na 2 SO 4 Ba. SO 4 + Na. Cl 10. Copper(II)nitrate Copper(II)oxide + Nitrogen dioxide + Oxygen Cu(NO 3)2 Cu. O + NO 2 + O 2

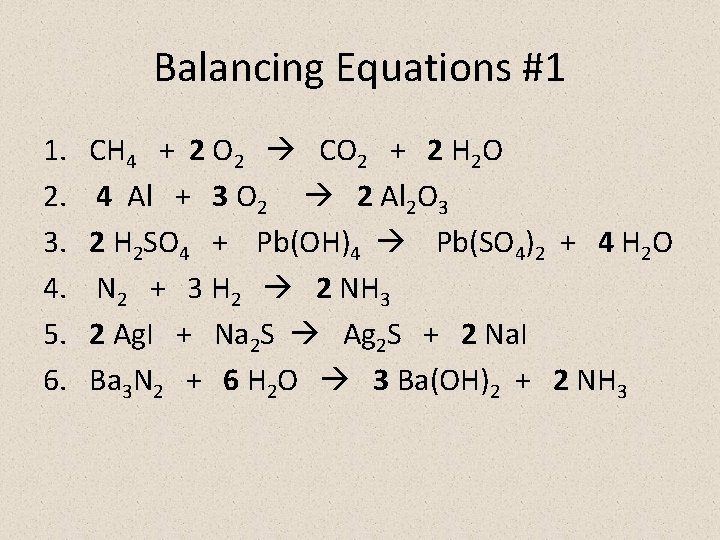
Balancing Equations #1 1. 2. 3. 4. 5. 6. CH 4 + 2 O 2 CO 2 + 2 H 2 O 4 Al + 3 O 2 2 Al 2 O 3 2 H 2 SO 4 + Pb(OH)4 Pb(SO 4)2 + 4 H 2 O N 2 + 3 H 2 2 NH 3 2 Ag. I + Na 2 S Ag 2 S + 2 Na. I Ba 3 N 2 + 6 H 2 O 3 Ba(OH)2 + 2 NH 3

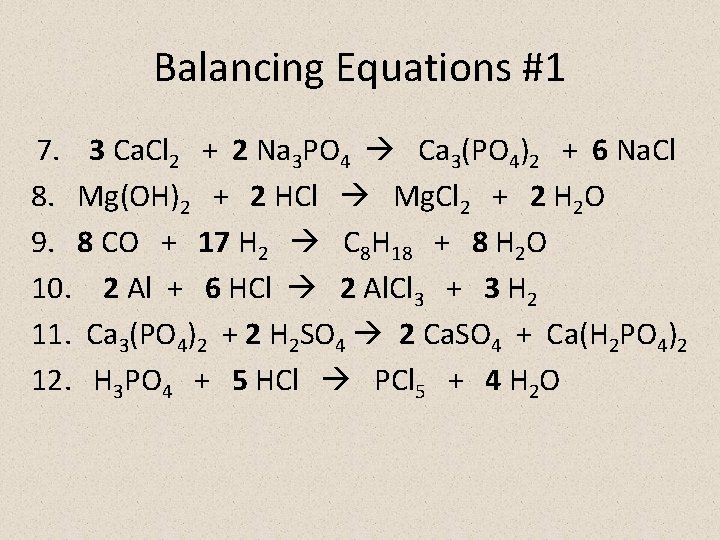
Balancing Equations #1 7. 3 Ca. Cl 2 + 2 Na 3 PO 4 Ca 3(PO 4)2 + 6 Na. Cl 8. Mg(OH)2 + 2 HCl Mg. Cl 2 + 2 H 2 O 9. 8 CO + 17 H 2 C 8 H 18 + 8 H 2 O 10. 2 Al + 6 HCl 2 Al. Cl 3 + 3 H 2 11. Ca 3(PO 4)2 + 2 H 2 SO 4 2 Ca. SO 4 + Ca(H 2 PO 4)2 12. H 3 PO 4 + 5 HCl PCl 5 + 4 H 2 O

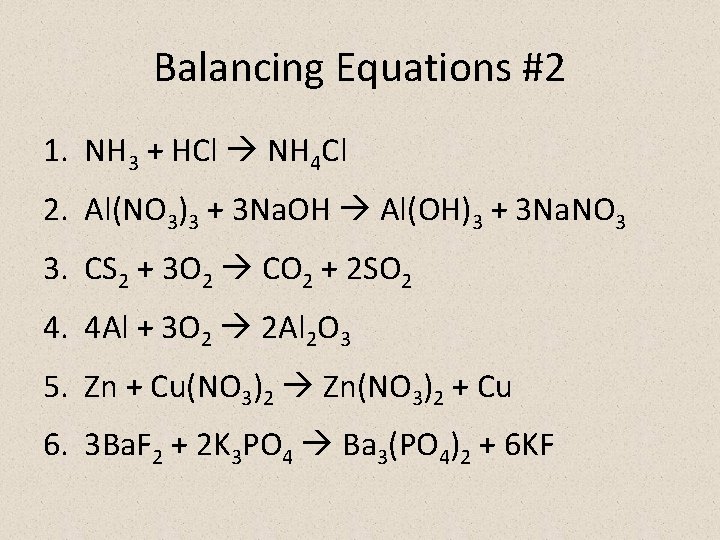
Balancing Equations #2 1. NH 3 + HCl NH 4 Cl 2. Al(NO 3)3 + 3 Na. OH Al(OH)3 + 3 Na. NO 3 3. CS 2 + 3 O 2 CO 2 + 2 SO 2 4. 4 Al + 3 O 2 2 Al 2 O 3 5. Zn + Cu(NO 3)2 Zn(NO 3)2 + Cu 6. 3 Ba. F 2 + 2 K 3 PO 4 Ba 3(PO 4)2 + 6 KF

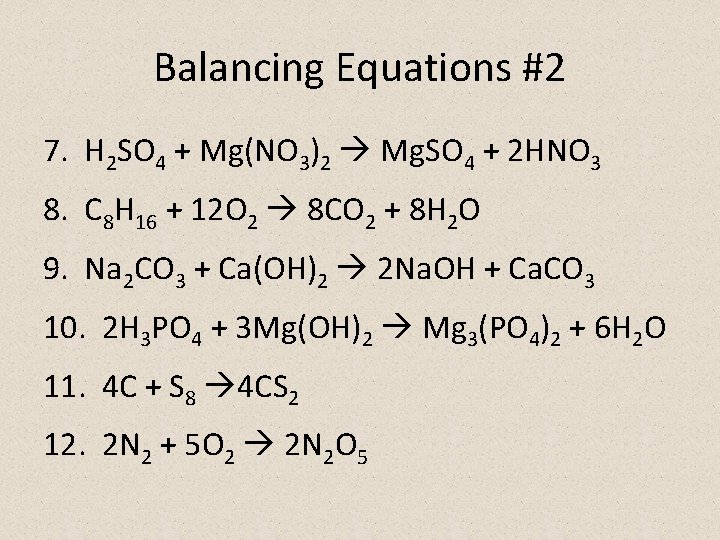
Balancing Equations #2 7. H 2 SO 4 + Mg(NO 3)2 Mg. SO 4 + 2 HNO 3 8. C 8 H 16 + 12 O 2 8 CO 2 + 8 H 2 O 9. Na 2 CO 3 + Ca(OH)2 2 Na. OH + Ca. CO 3 10. 2 H 3 PO 4 + 3 Mg(OH)2 Mg 3(PO 4)2 + 6 H 2 O 11. 4 C + S 8 4 CS 2 12. 2 N 2 + 5 O 2 2 N 2 O 5

Word Equations (Extra Practice) 1. 2. 3. 4. 5. 6. 7. 8. Zn + Pb(NO 3)2 Zn(NO 3)2 + Pb 2 Al. Br 3 + 3 Cl 2 2 Al. Cl 3 + 3 Br 2 2 Na 3 PO 4 + 3 Ca. Cl 2 Ca 3(PO 4)2 + 6 Na. Cl 2 KCl. O 3 2 KCl + 3 O 2 2 Al + 6 HCl 2 Al. Cl 3 + 3 H 2 3 Ca(OH)2 + 2 H 3 PO 4 Ca 3(PO 4)2 + 6 H 2 O Cu + 2 H 2 SO 4 Cu. SO 4 + 2 H 2 O + SO 2 2 H 2 + 2 NO 2 H 2 O + N 2

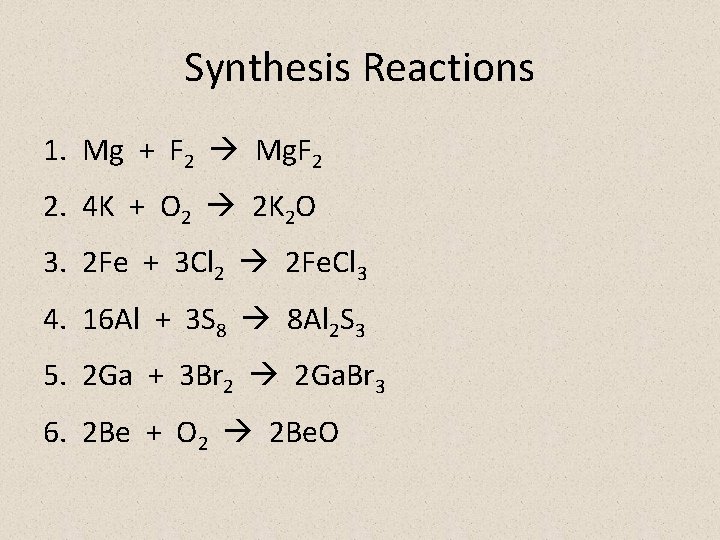
Synthesis Reactions 1. Mg + F 2 Mg. F 2 2. 4 K + O 2 2 K 2 O 3. 2 Fe + 3 Cl 2 2 Fe. Cl 3 4. 16 Al + 3 S 8 8 Al 2 S 3 5. 2 Ga + 3 Br 2 2 Ga. Br 3 6. 2 Be + O 2 2 Be. O

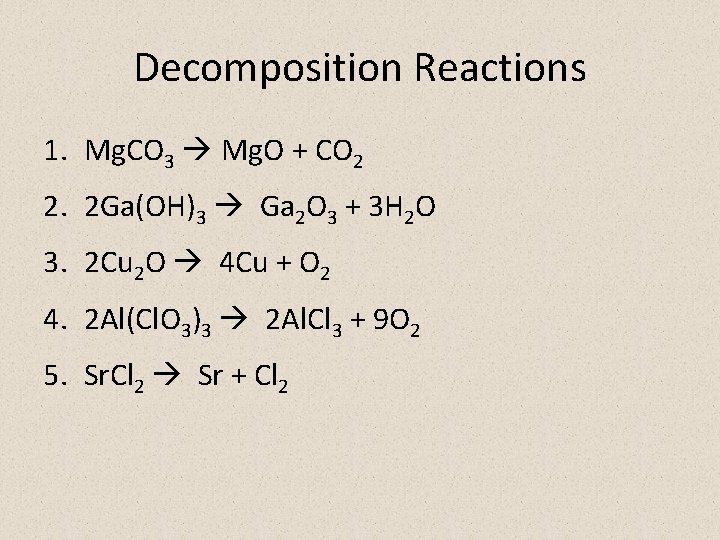
Decomposition Reactions 1. Mg. CO 3 Mg. O + CO 2 2. 2 Ga(OH)3 Ga 2 O 3 + 3 H 2 O 3. 2 Cu 2 O 4 Cu + O 2 4. 2 Al(Cl. O 3)3 2 Al. Cl 3 + 9 O 2 5. Sr. Cl 2 Sr + Cl 2

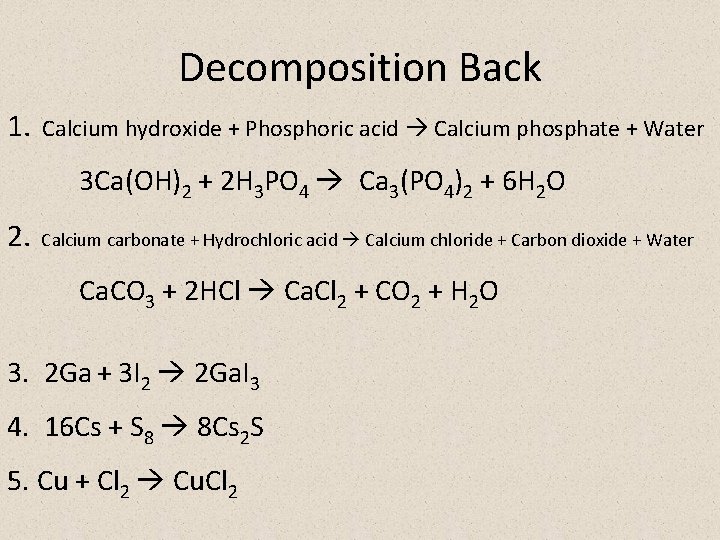
Decomposition Back 1. Calcium hydroxide + Phosphoric acid Calcium phosphate + Water 3 Ca(OH)2 + 2 H 3 PO 4 Ca 3(PO 4)2 + 6 H 2 O 2. Calcium carbonate + Hydrochloric acid Calcium chloride + Carbon dioxide + Water Ca. CO 3 + 2 HCl Ca. Cl 2 + CO 2 + H 2 O 3. 2 Ga + 3 I 2 2 Ga. I 3 4. 16 Cs + S 8 8 Cs 2 S 5. Cu + Cl 2 Cu. Cl 2

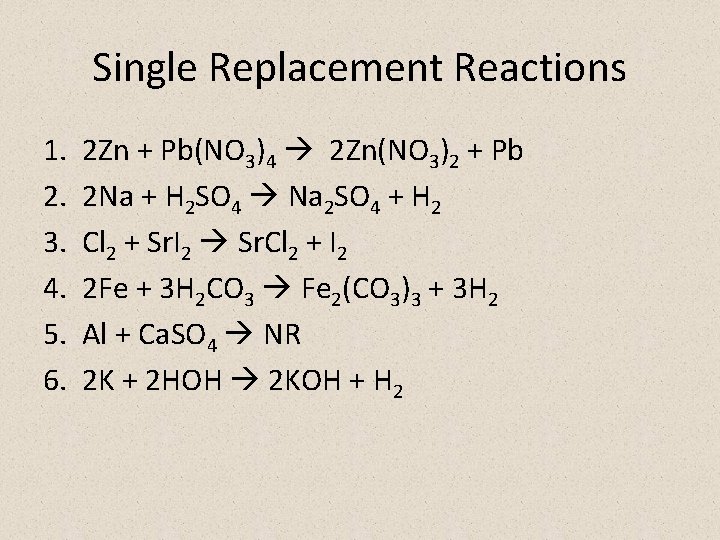
Single Replacement Reactions 1. 2. 3. 4. 5. 6. 2 Zn + Pb(NO 3)4 2 Zn(NO 3)2 + Pb 2 Na + H 2 SO 4 Na 2 SO 4 + H 2 Cl 2 + Sr. I 2 Sr. Cl 2 + I 2 2 Fe + 3 H 2 CO 3 Fe 2(CO 3)3 + 3 H 2 Al + Ca. SO 4 NR 2 K + 2 HOH 2 KOH + H 2

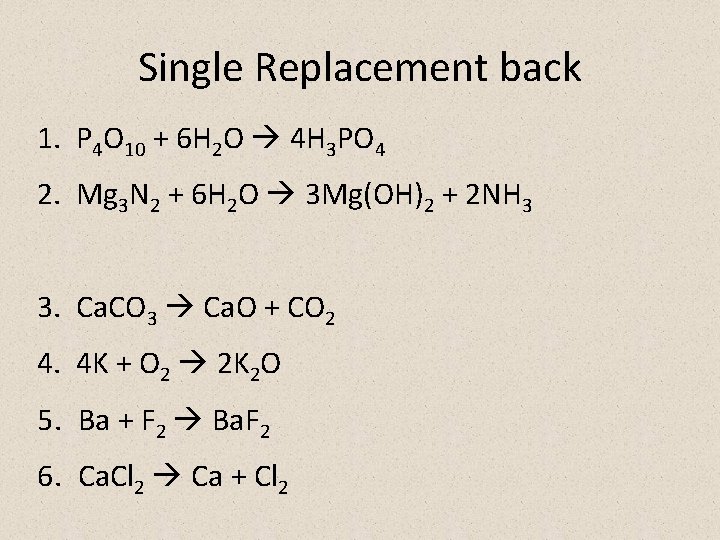
Single Replacement back 1. P 4 O 10 + 6 H 2 O 4 H 3 PO 4 2. Mg 3 N 2 + 6 H 2 O 3 Mg(OH)2 + 2 NH 3 3. Ca. CO 3 Ca. O + CO 2 4. 4 K + O 2 2 K 2 O 5. Ba + F 2 Ba. F 2 6. Ca. Cl 2 Ca + Cl 2

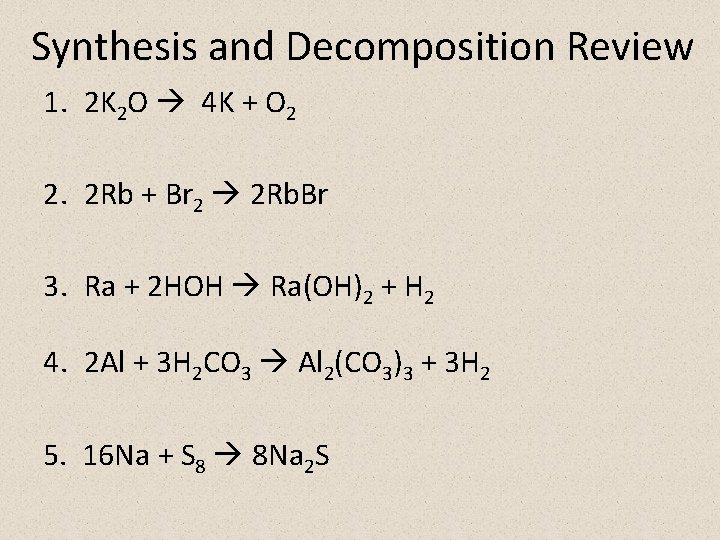
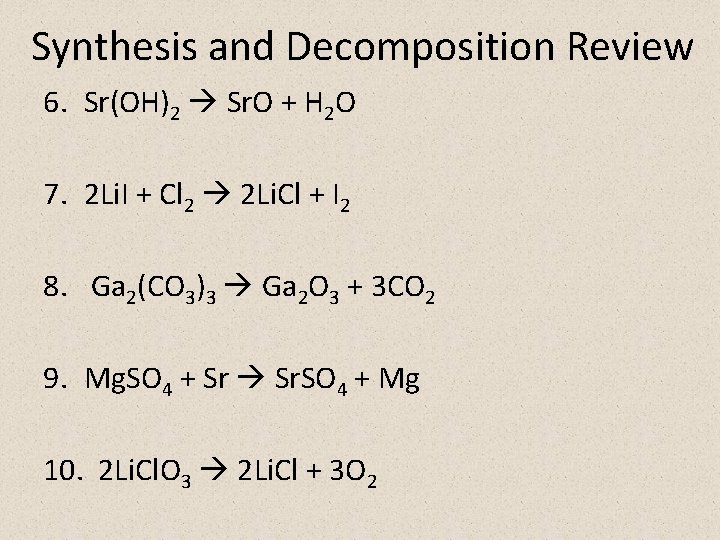
Synthesis and Decomposition Review 1. 2 K 2 O 4 K + O 2 2. 2 Rb + Br 2 2 Rb. Br 3. Ra + 2 HOH Ra(OH)2 + H 2 4. 2 Al + 3 H 2 CO 3 Al 2(CO 3)3 + 3 H 2 5. 16 Na + S 8 8 Na 2 S

Synthesis and Decomposition Review 6. Sr(OH)2 Sr. O + H 2 O 7. 2 Li. I + Cl 2 2 Li. Cl + I 2 8. Ga 2(CO 3)3 Ga 2 O 3 + 3 CO 2 9. Mg. SO 4 + Sr Sr. SO 4 + Mg 10. 2 Li. Cl. O 3 2 Li. Cl + 3 O 2

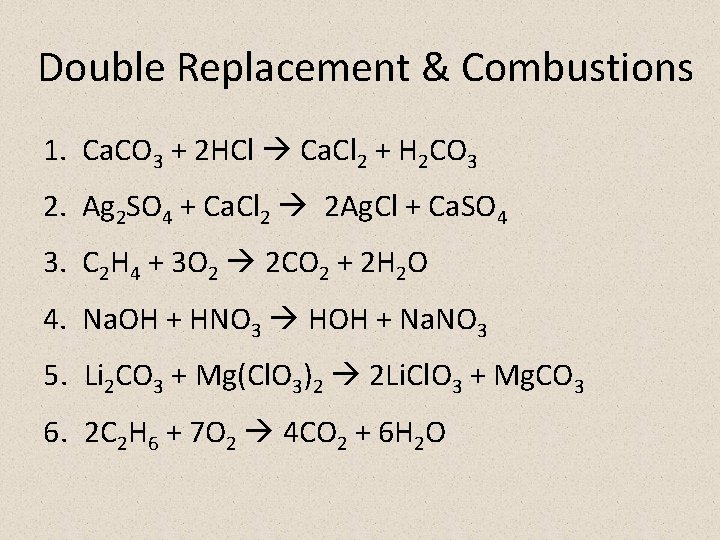
Double Replacement & Combustions 1. Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3 2. Ag 2 SO 4 + Ca. Cl 2 2 Ag. Cl + Ca. SO 4 3. C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O 4. Na. OH + HNO 3 HOH + Na. NO 3 5. Li 2 CO 3 + Mg(Cl. O 3)2 2 Li. Cl. O 3 + Mg. CO 3 6. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O

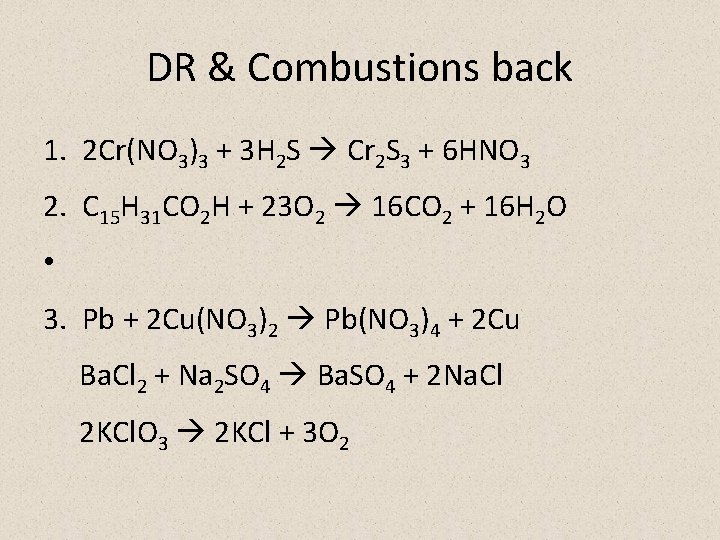
DR & Combustions back 1. 2 Cr(NO 3)3 + 3 H 2 S Cr 2 S 3 + 6 HNO 3 2. C 15 H 31 CO 2 H + 23 O 2 16 CO 2 + 16 H 2 O • 3. Pb + 2 Cu(NO 3)2 Pb(NO 3)4 + 2 Cu Ba. Cl 2 + Na 2 SO 4 Ba. SO 4 + 2 Na. Cl 2 KCl. O 3 2 KCl + 3 O 2

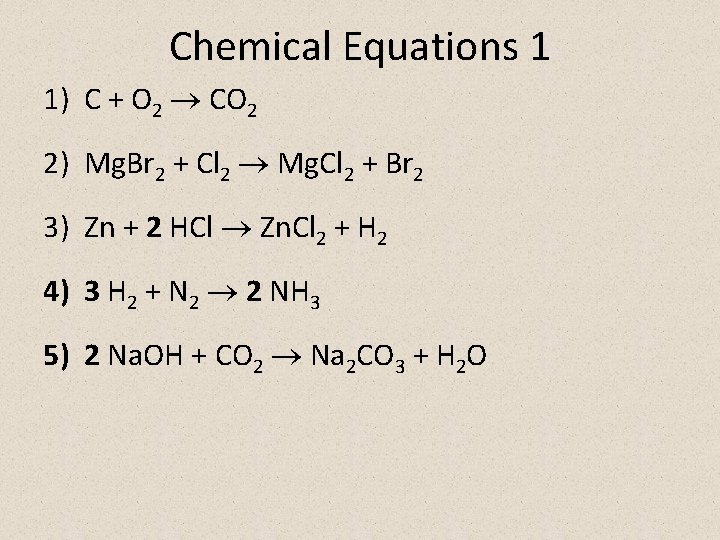
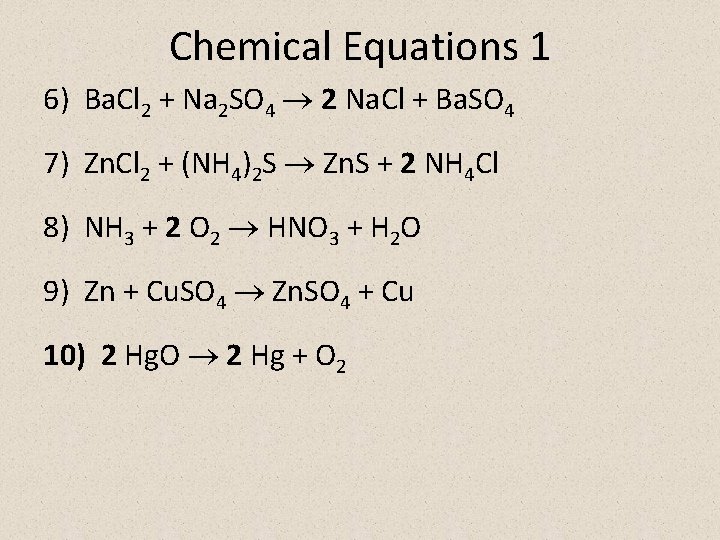
Chemical Equations 1 1) C + O 2 CO 2 2) Mg. Br 2 + Cl 2 Mg. Cl 2 + Br 2 3) Zn + 2 HCl Zn. Cl 2 + H 2 4) 3 H 2 + N 2 2 NH 3 5) 2 Na. OH + CO 2 Na 2 CO 3 + H 2 O

Chemical Equations 1 6) Ba. Cl 2 + Na 2 SO 4 2 Na. Cl + Ba. SO 4 7) Zn. Cl 2 + (NH 4)2 S Zn. S + 2 NH 4 Cl 8) NH 3 + 2 O 2 HNO 3 + H 2 O 9) Zn + Cu. SO 4 Zn. SO 4 + Cu 10) 2 Hg. O 2 Hg + O 2

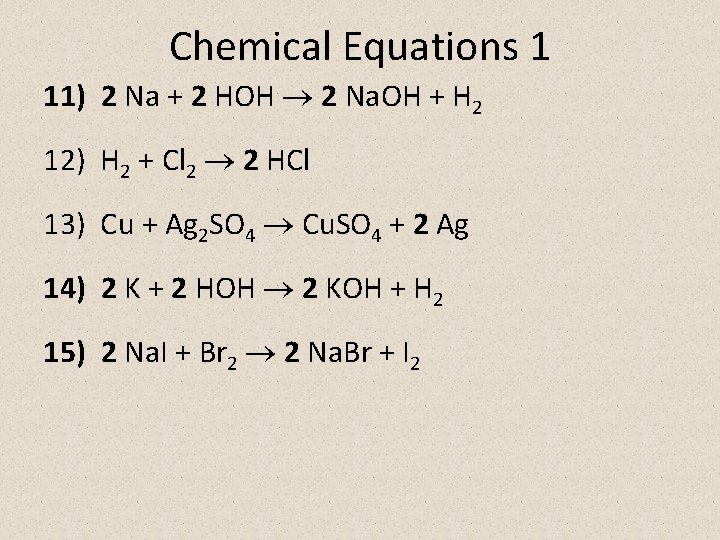
Chemical Equations 1 11) 2 Na + 2 HOH 2 Na. OH + H 2 12) H 2 + Cl 2 2 HCl 13) Cu + Ag 2 SO 4 Cu. SO 4 + 2 Ag 14) 2 K + 2 HOH 2 KOH + H 2 15) 2 Na. I + Br 2 2 Na. Br + I 2

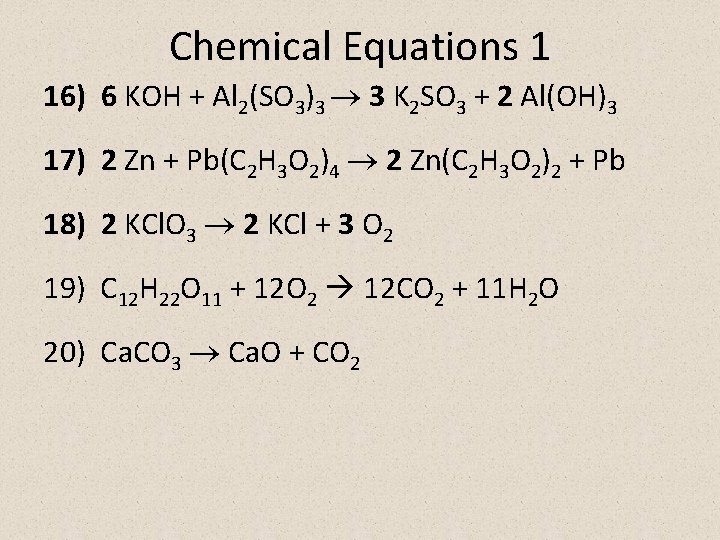
Chemical Equations 1 16) 6 KOH + Al 2(SO 3)3 3 K 2 SO 3 + 2 Al(OH)3 17) 2 Zn + Pb(C 2 H 3 O 2)4 2 Zn(C 2 H 3 O 2)2 + Pb 18) 2 KCl. O 3 2 KCl + 3 O 2 19) C 12 H 22 O 11 + 12 O 2 12 CO 2 + 11 H 2 O 20) Ca. CO 3 Ca. O + CO 2

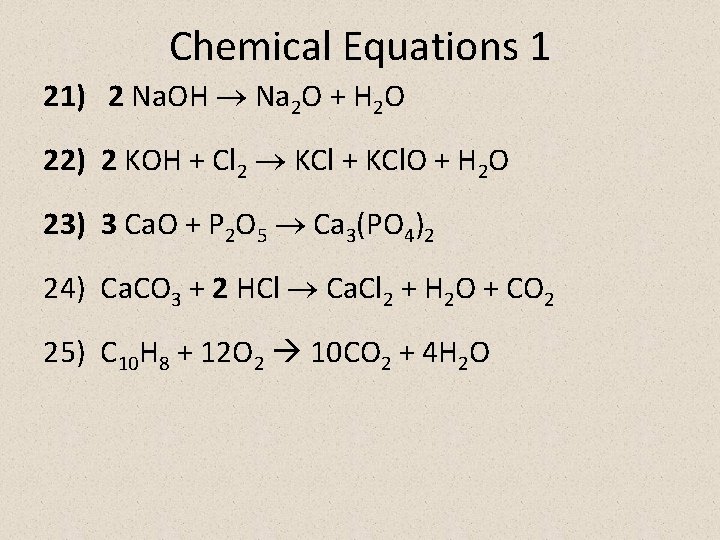
Chemical Equations 1 21) 2 Na. OH Na 2 O + H 2 O 22) 2 KOH + Cl 2 KCl + KCl. O + H 2 O 23) 3 Ca. O + P 2 O 5 Ca 3(PO 4)2 24) Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 O + CO 2 25) C 10 H 8 + 12 O 2 10 CO 2 + 4 H 2 O

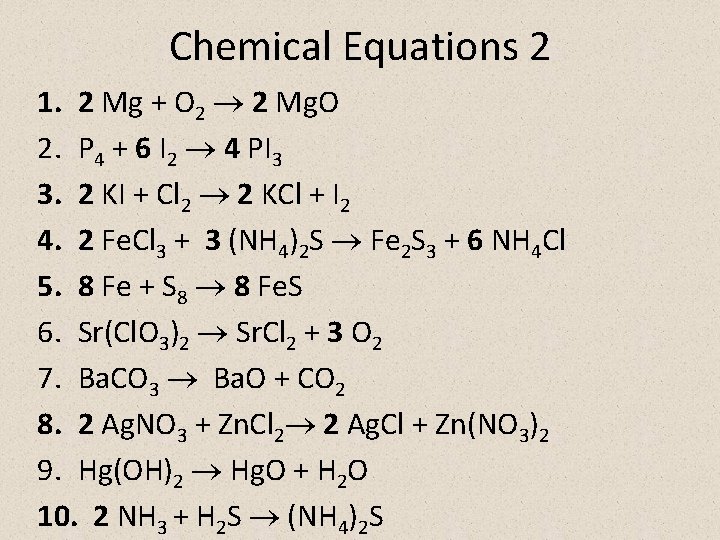
Chemical Equations 2 1. 2 Mg + O 2 2 Mg. O 2. P 4 + 6 I 2 4 PI 3 3. 2 KI + Cl 2 2 KCl + I 2 4. 2 Fe. Cl 3 + 3 (NH 4)2 S Fe 2 S 3 + 6 NH 4 Cl 5. 8 Fe + S 8 8 Fe. S 6. Sr(Cl. O 3)2 Sr. Cl 2 + 3 O 2 7. Ba. CO 3 Ba. O + CO 2 8. 2 Ag. NO 3 + Zn. Cl 2 2 Ag. Cl + Zn(NO 3)2 9. Hg(OH)2 Hg. O + H 2 O 10. 2 NH 3 + H 2 S (NH 4)2 S

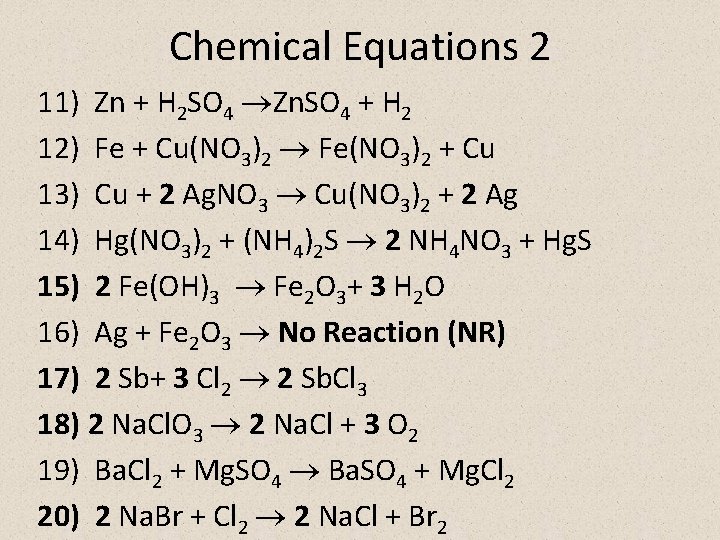
Chemical Equations 2 11) Zn + H 2 SO 4 Zn. SO 4 + H 2 12) Fe + Cu(NO 3)2 Fe(NO 3)2 + Cu 13) Cu + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag 14) Hg(NO 3)2 + (NH 4)2 S 2 NH 4 NO 3 + Hg. S 15) 2 Fe(OH)3 Fe 2 O 3+ 3 H 2 O 16) Ag + Fe 2 O 3 No Reaction (NR) 17) 2 Sb+ 3 Cl 2 2 Sb. Cl 3 18) 2 Na. Cl. O 3 2 Na. Cl + 3 O 2 19) Ba. Cl 2 + Mg. SO 4 Ba. SO 4 + Mg. Cl 2 20) 2 Na. Br + Cl 2 2 Na. Cl + Br 2

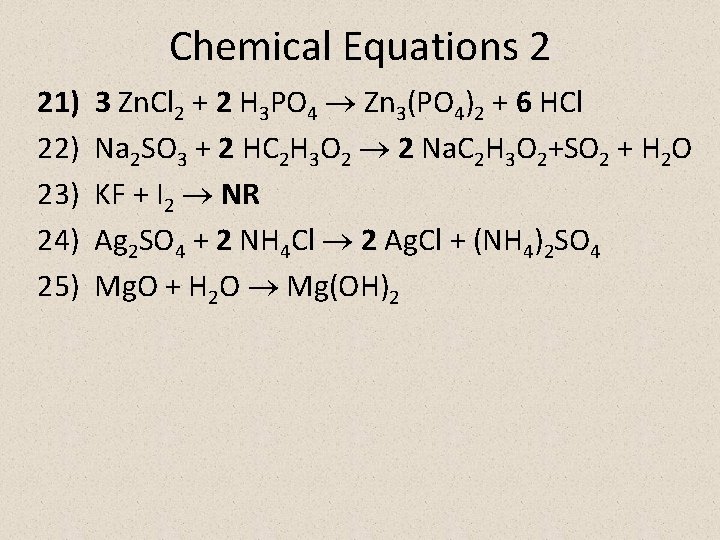
Chemical Equations 2 21) 22) 23) 24) 25) 3 Zn. Cl 2 + 2 H 3 PO 4 Zn 3(PO 4)2 + 6 HCl Na 2 SO 3 + 2 HC 2 H 3 O 2 2 Na. C 2 H 3 O 2+SO 2 + H 2 O KF + I 2 NR Ag 2 SO 4 + 2 NH 4 Cl 2 Ag. Cl + (NH 4)2 SO 4 Mg. O + H 2 O Mg(OH)2

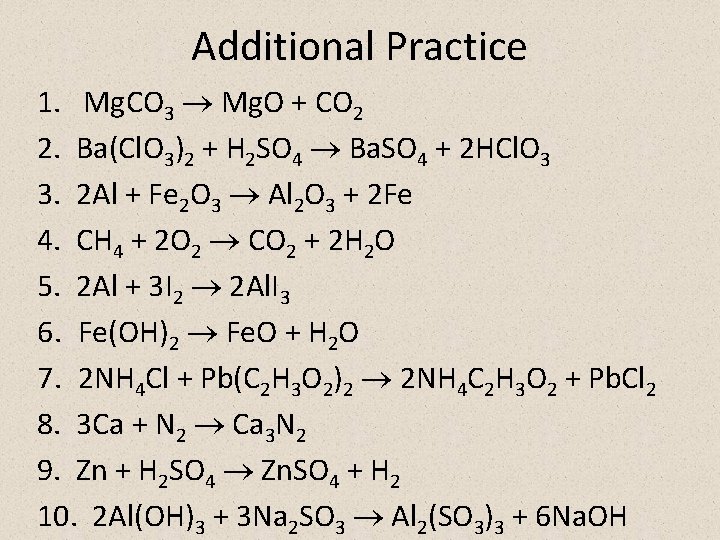
Additional Practice 1. Mg. CO 3 Mg. O + CO 2 2. Ba(Cl. O 3)2 + H 2 SO 4 Ba. SO 4 + 2 HCl. O 3 3. 2 Al + Fe 2 O 3 Al 2 O 3 + 2 Fe 4. CH 4 + 2 O 2 CO 2 + 2 H 2 O 5. 2 Al + 3 I 2 2 Al. I 3 6. Fe(OH)2 Fe. O + H 2 O 7. 2 NH 4 Cl + Pb(C 2 H 3 O 2)2 2 NH 4 C 2 H 3 O 2 + Pb. Cl 2 8. 3 Ca + N 2 Ca 3 N 2 9. Zn + H 2 SO 4 Zn. SO 4 + H 2 10. 2 Al(OH)3 + 3 Na 2 SO 3 Al 2(SO 3)3 + 6 Na. OH

Additional Practice 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O 2 Na + 2 HOH H 2 + 2 Na. OH Ca. Cl 2 + I 2 NR 2 Ag + Br 2 2 Ag. Br Zn + Cu. SO 4 Zn. SO 4 + Cu 2 Al(Cl. O 3)3 2 Al. Cl 3 + 9 O 2 C 3 H 4 + 4 O 2 3 CO 2 + 2 H 2 O Fe. Br 3 + Na 3 PO 4 Fe. PO 4 + 3 Na. Br 4 Cu + O 2 2 Cu 2 O 2 C 4 H 6 + 11 O 2 8 CO 2 + 6 H 2 O

Additional Practice 21. 22. 23. 24. 25. 2 Na. Br + Cl 2 2 Na. Cl + Br 2 16 Ga + 3 S 8 8 Ga 2 S 3 C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Ca(NO 3)2 + 2 KOH Ca(OH)2 + 2 KNO 3 2 Al(Cl. O 3)3 2 Al. Cl 3 + 9 O 2

9 Chapter Test 1. D 2. B 3. A 4. C 5. A 6. C 7. A (Synthesis) 8. C 9. A 10. B • • • Synthesis Decomposition Double Replacement Decomposition Single Replacement

9 Chapter Test 16. Water + Calcium hydroxide + hydrogen 2 H 2 O + Ca Ca(OH)2 + H 2 17. Bromine + Nitrogen tribromide 3 Br 2 + N 2 2 NBr 3 18. Ammonia + Hydrogen sulfide ammonium sulfide 2 NH 3 + H 2 S (NH 4)2 S

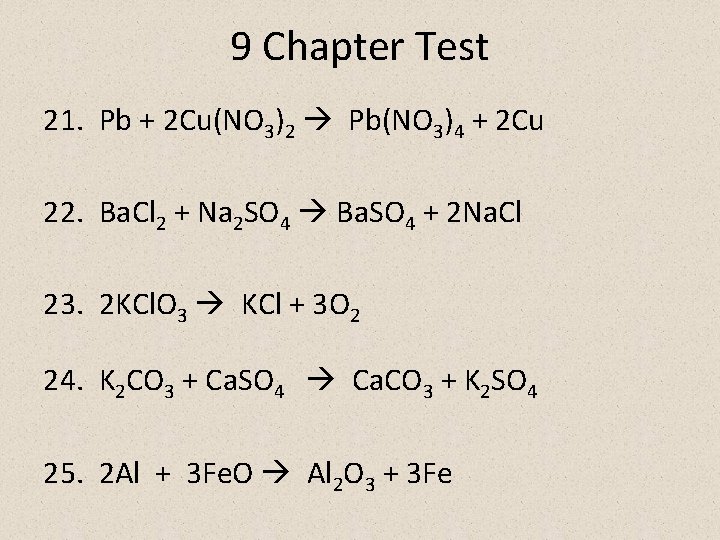
9 Chapter Test 21. Pb + 2 Cu(NO 3)2 Pb(NO 3)4 + 2 Cu 22. Ba. Cl 2 + Na 2 SO 4 Ba. SO 4 + 2 Na. Cl 23. 2 KCl. O 3 KCl + 3 O 2 24. K 2 CO 3 + Ca. SO 4 Ca. CO 3 + K 2 SO 4 25. 2 Al + 3 Fe. O Al 2 O 3 + 3 Fe