Molecular Spectroscopy 1 INTRODUCTION What is molecular Spectroscopy









- Slides: 60

Molecular Spectroscopy 1

INTRODUCTION • What is molecular Spectroscopy? ? • Applications: (in petroleum chemistry) • Components of Spectroscopy – Light: electromagnetic radiations – Molecular behavior • classification of spectroscopy • Techniques involved • Analysis 2

What is spectroscopy? ? • Spectroscopy is the study of interaction of light with matter • More specifically, it is the study of properties of matter through its interaction with different frequency component of the light (electromagnetic radiation) • With light we are not looking the molecule directly……instead we observe the INTERACTION of light with different types of molecules_______ Each type of spectroscopy (different light frequency)-----gives a picture (our result), known as SPECTRUM---- which gives lots of informations!!! 3

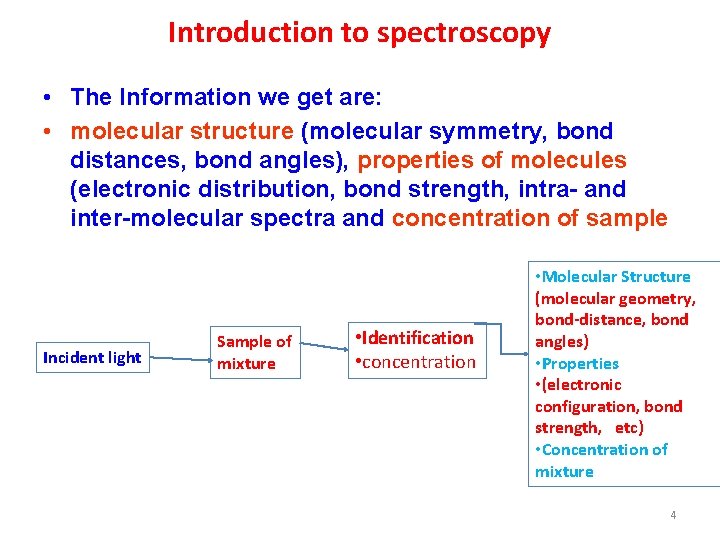
Introduction to spectroscopy • The Information we get are: • molecular structure (molecular symmetry, bond distances, bond angles), properties of molecules (electronic distribution, bond strength, intra- and inter-molecular spectra and concentration of sample Incident light Sample of mixture • Identification • concentration • Molecular Structure (molecular geometry, bond distance, bond angles) • Properties • (electronic configuration, bond strength, etc) • Concentration of mixture 4

Applications of spectroscopy • Spectroscopic techniques are some of the most widely used analytical methods in the world today. • Properties of organic, inorganic molecules • Petroleum products are hydrocarbons, i. e. , they contain at least hydrogen and carbon— often other elements such as nitrogen, oxygen, halogens, phosphorus and sulfur. Most of the bonding involved in organic compounds is covalent. 5

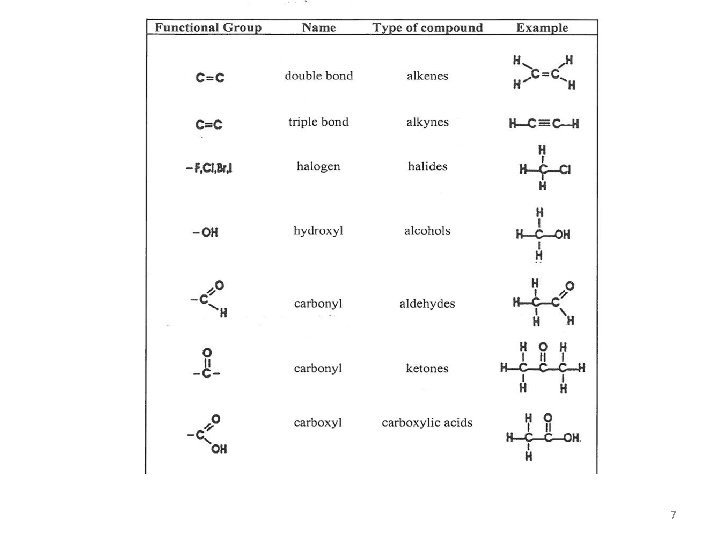
Applications of spectroscopy • The physical and chemical properties of these compounds are highly structure related. • Attachment of small groups of atoms known as functional groups to a hydrocarbon can yield a new substance with properties at least in part characteristic of that group. • What type of hydrocarbon is present in this mixture? ? Molecular Structure (bond distance, bond angles, total number and types of bonds present in the molecule), functional group attached 6

7

The immediate questions we want to address are: • • • What is LIGHT? ? What does light do to sample? ? What is molecular sample? How do we produce a spectrum for a sample? What exactly a spectrum can give informations? 8

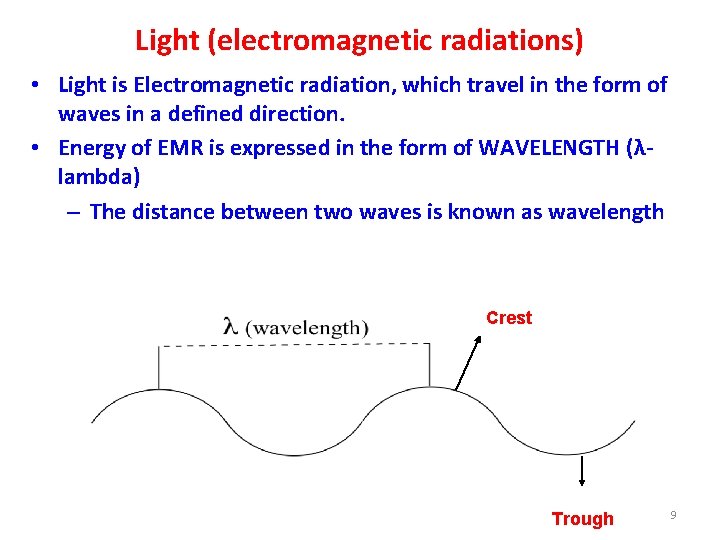
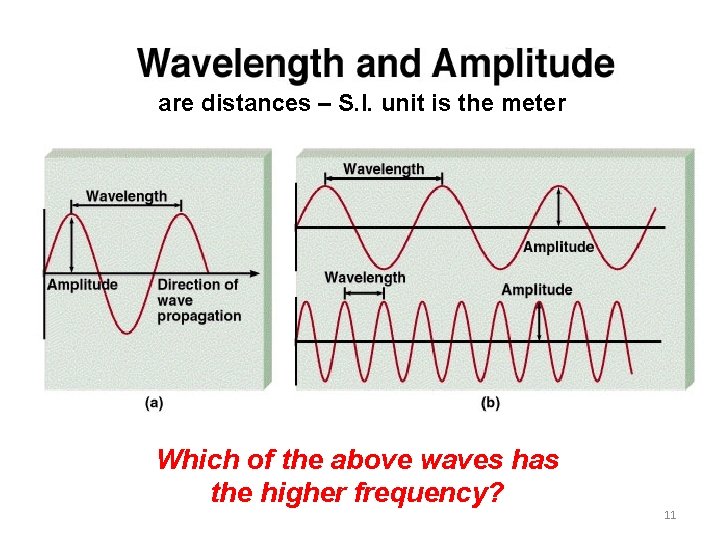
Light (electromagnetic radiations) • Light is Electromagnetic radiation, which travel in the form of waves in a defined direction. • Energy of EMR is expressed in the form of WAVELENGTH (λ lambda) – The distance between two waves is known as wavelength Crest Trough 9

Electromagnetic Radiation • Visible light is just one form of electromagnetic radiation. • Light travels in space as a wave. • In vacuum, speed of light is constant and given the symbol “c”, c = 3. 00 x 108 m/s. • Light waves have amplitude, frequency, and wavelength. • Wavelength ( ) : distance between consecutive crests. • Frequency ( ) : number of waves that pass a given point in one second (SI Unit is s 1 or Hz). c = and = c/ 10

are distances – S. I. unit is the meter Which of the above waves has the higher frequency? 11

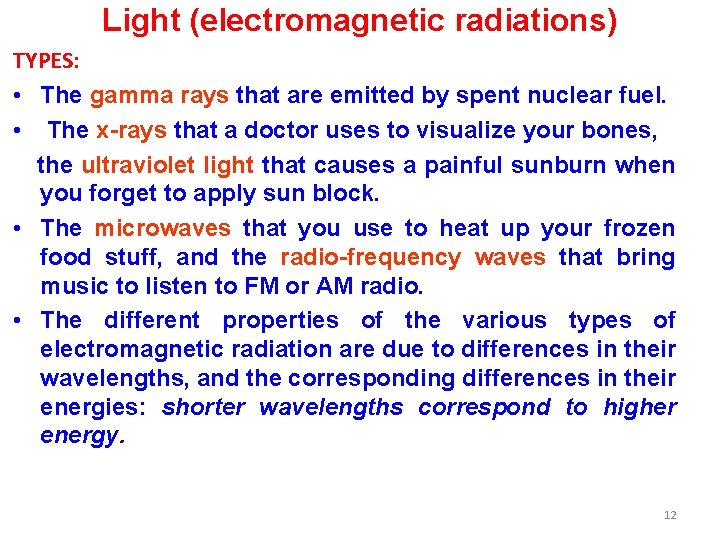
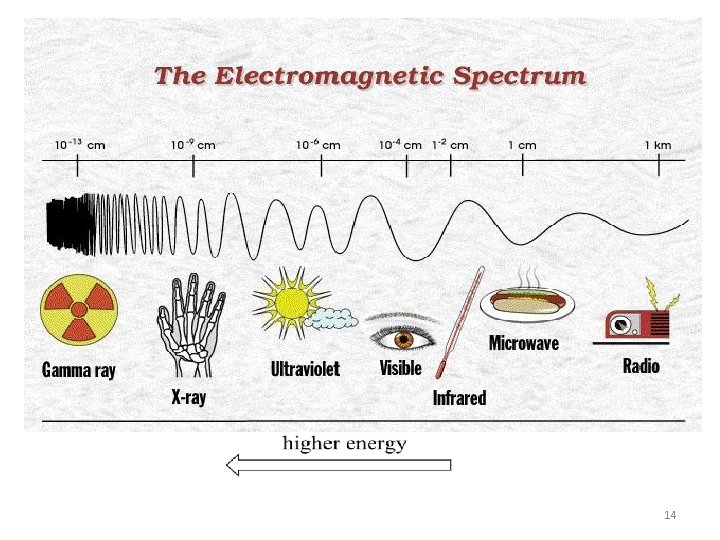
Light (electromagnetic radiations) TYPES: • The gamma rays that are emitted by spent nuclear fuel. • The x-rays that a doctor uses to visualize your bones, the ultraviolet light that causes a painful sunburn when you forget to apply sun block. • The microwaves that you use to heat up your frozen food stuff, and the radio-frequency waves that bring music to listen to FM or AM radio. • The different properties of the various types of electromagnetic radiation are due to differences in their wavelengths, and the corresponding differences in their energies: shorter wavelengths correspond to higher energy. 12

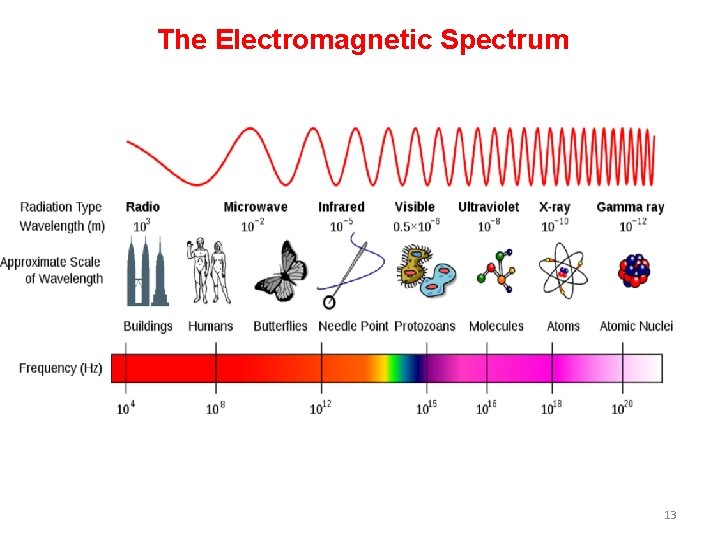
The Electromagnetic Spectrum 13

14

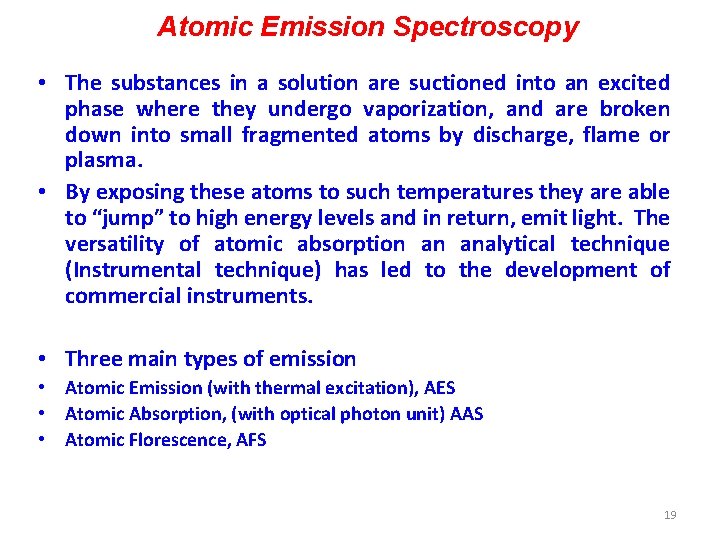
What does light to do to sample? ? • Basically, we characterize how a sample modifies light entering in it. E. g. • Can Absorb light (absorption spectroscopy) • Can emit light (emission spectroscopy) 15

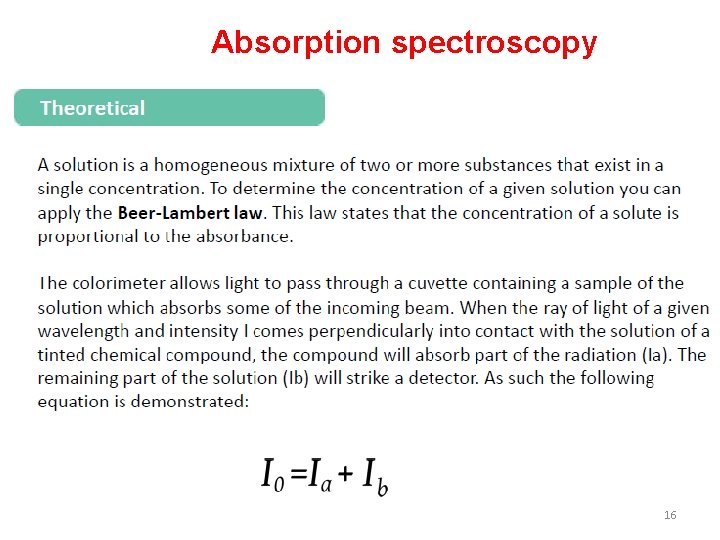
Absorption spectroscopy 16

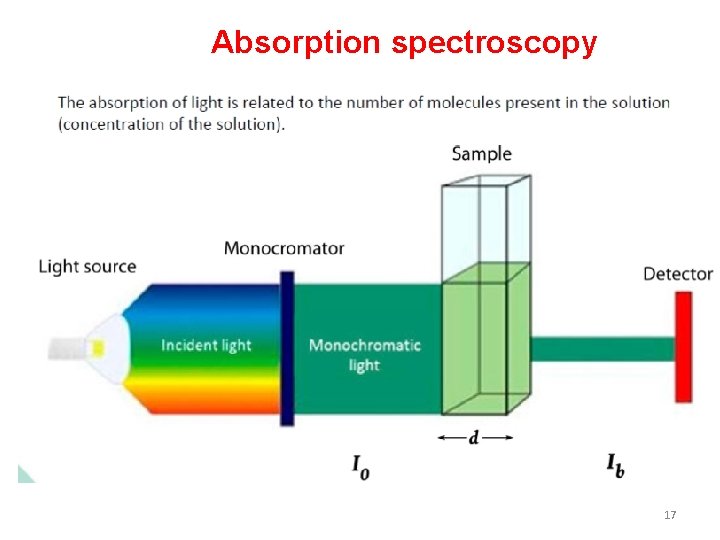
Absorption spectroscopy 17

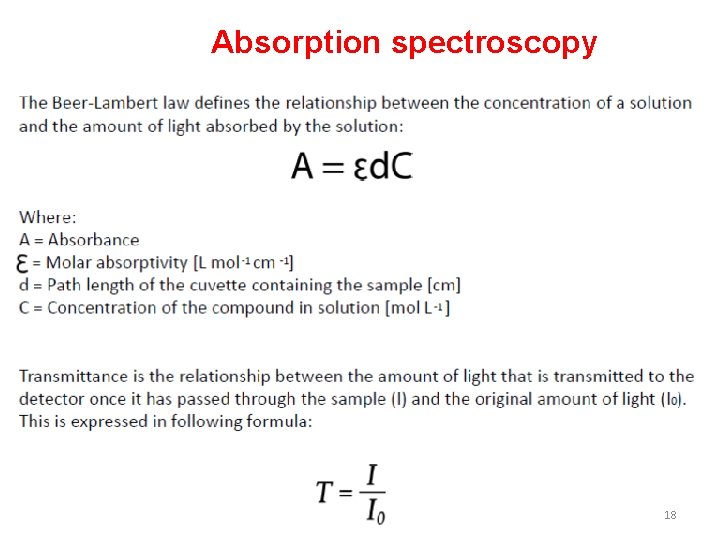
Absorption spectroscopy 18

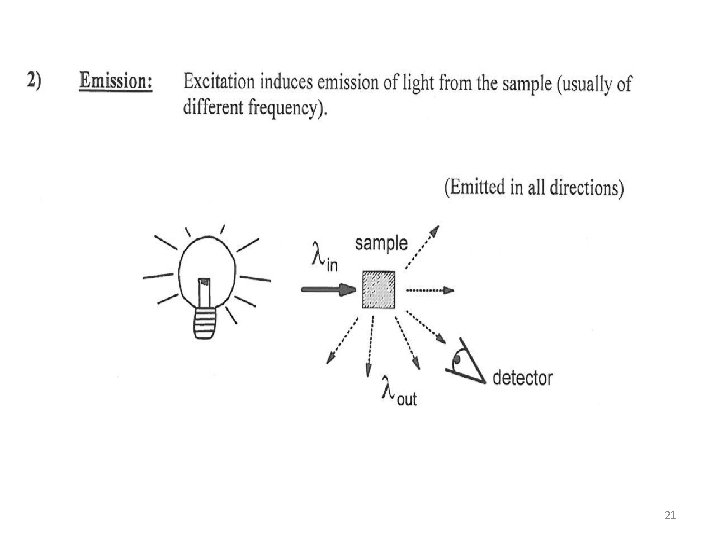
Atomic Emission Spectroscopy • The substances in a solution are suctioned into an excited phase where they undergo vaporization, and are broken down into small fragmented atoms by discharge, flame or plasma. • By exposing these atoms to such temperatures they are able to “jump” to high energy levels and in return, emit light. The versatility of atomic absorption an analytical technique (Instrumental technique) has led to the development of commercial instruments. • Three main types of emission • Atomic Emission (with thermal excitation), AES • Atomic Absorption, (with optical photon unit) AAS • Atomic Florescence, AFS 19

Principle of Emission Spectroscopy • As with fluorescence, the atomic emission is a result of electrons dropping from an excited state to lower states. Following atomization, a small percentage of the atoms absorb sufficient energy from the flame so as to be promoted to an excited state. As with molecules in fluorescence, these atoms quickly return to a lower state, and light corresponding to the energy that is lost in the process is generated. It is this light that our eye perceives. 20

21

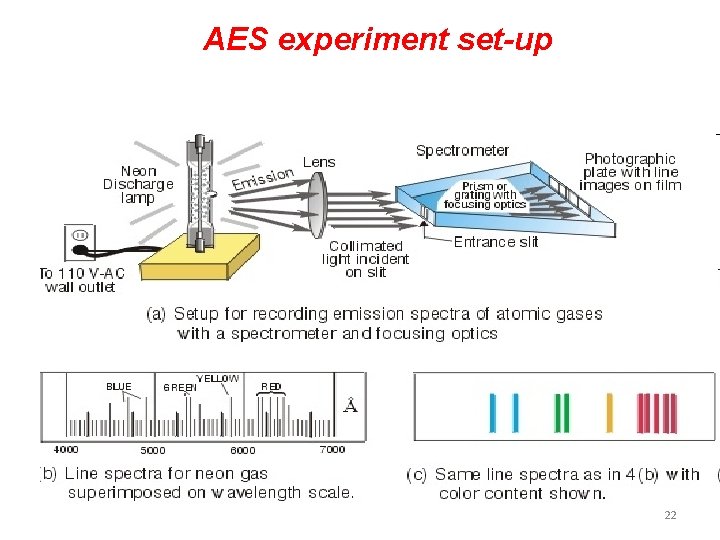
AES experiment set-up 22

What is molecular sample? • The electrons and nuclei of atoms and molecules may exist in only certain specific energy levels. • They can absorb energy and get excited to the higher energy levels example: diatomic molecule 23

How do we produce a spectrum for a sample? the basic idea • In a spectroscopy experiment, electromagnetic radiation of a specified range of wavelengths is allowed to pass through a sample containing a compound of interest. • The sample molecules absorb energy from some of the wavelengths, and as a result jump from a low energy ‘ground state’ to some higher energy ‘excited state’. Other wavelengths are not absorbed by the sample molecule, so they pass on through. • A detector on the other side of the sample records which wavelengths were absorbed, and to what extent they were absorbed. 24

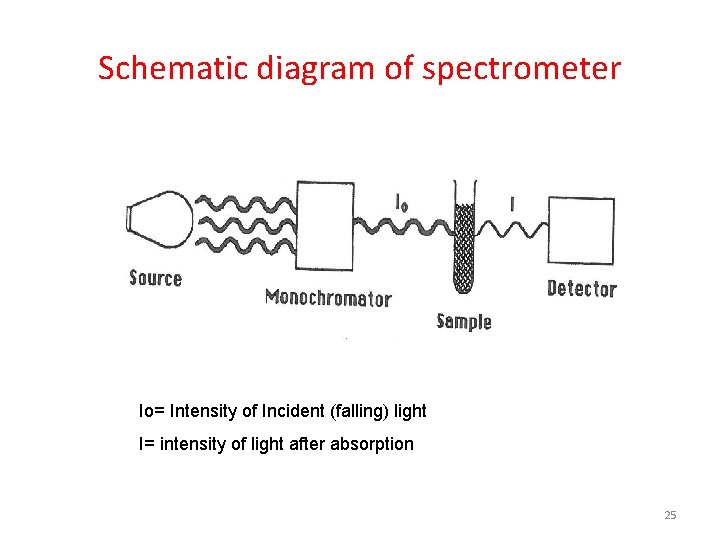
Schematic diagram of spectrometer Io= Intensity of Incident (falling) light I= intensity of light after absorption 25

How do we produce a spectrum for a sample? the basic idea • Here is the key to molecular spectroscopy: a given molecule will specifically absorb only those wavelengths which have energies that correspond to the energy difference of the transition that is occurring. Thus, if the transition involves the molecule jumping from ground state A to excited state B, with an energy difference of ΔE, the molecule will specifically absorb radiation with wavelength that corresponds to ΔE, while allowing other wavelengths to pass through unabsorbed. 26

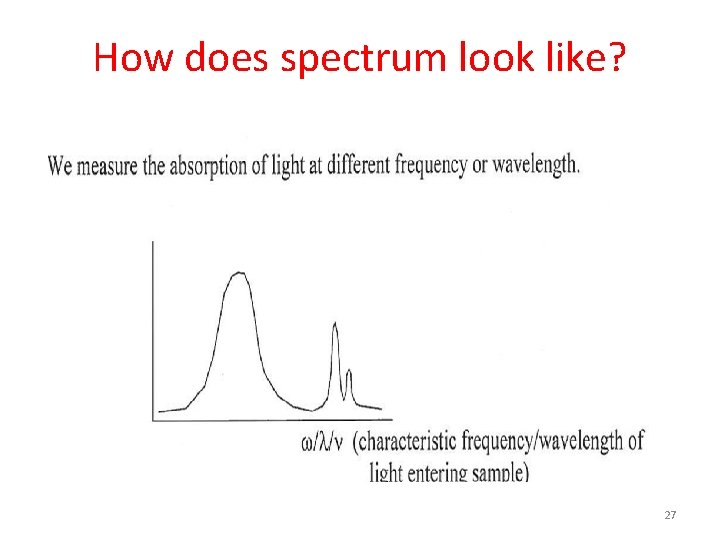
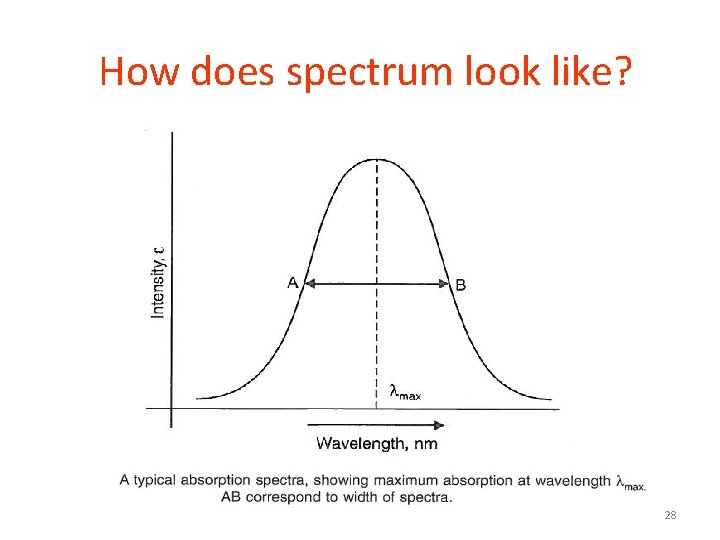
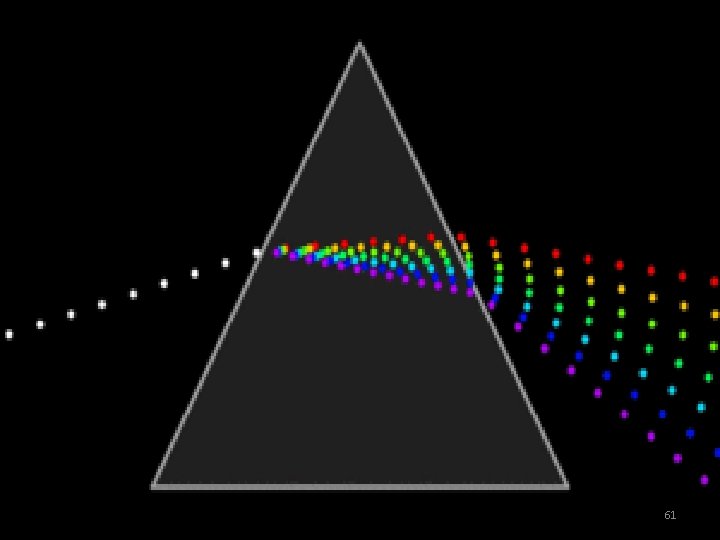
How does spectrum look like? 27

How does spectrum look like? 28

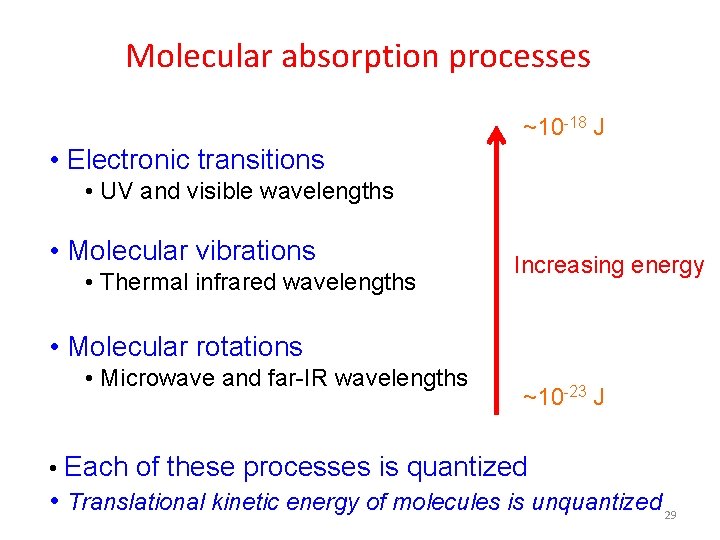
Molecular absorption processes ~10 -18 J • Electronic transitions • UV and visible wavelengths • Molecular vibrations • Thermal infrared wavelengths Increasing energy • Molecular rotations • Microwave and far-IR wavelengths ~10 -23 J • Each of these processes is quantized • Translational kinetic energy of molecules is unquantized 29

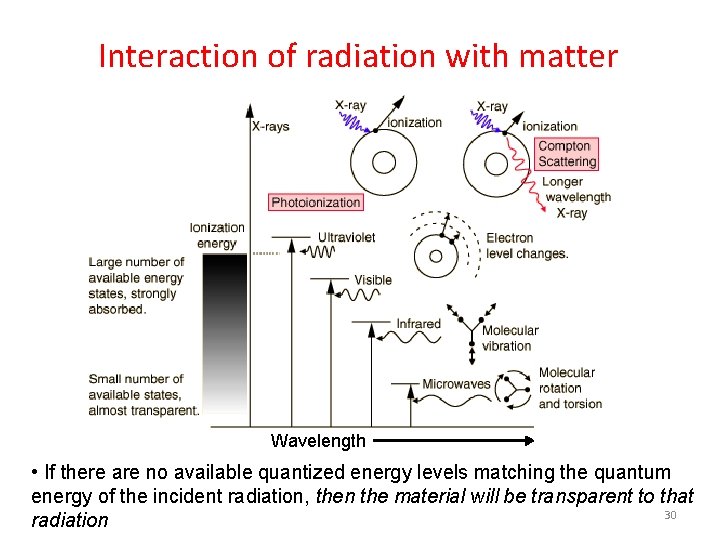
Interaction of radiation with matter Wavelength • If there are no available quantized energy levels matching the quantum energy of the incident radiation, then the material will be transparent to that 30 radiation

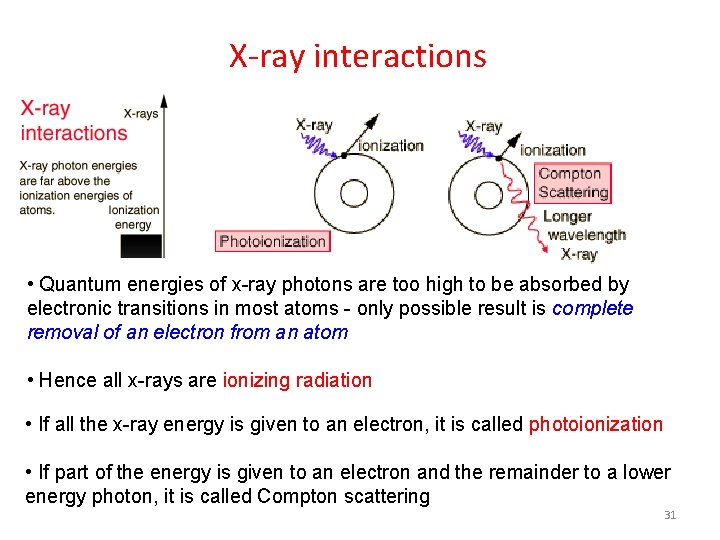
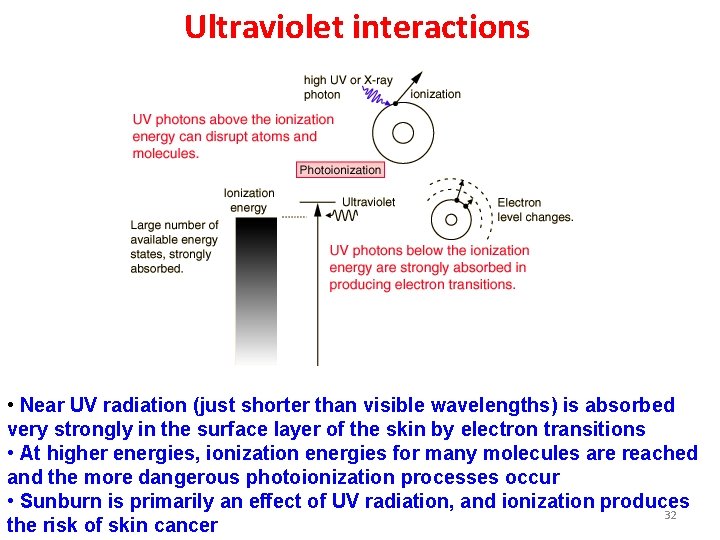
X-ray interactions • Quantum energies of x-ray photons are too high to be absorbed by electronic transitions in most atoms - only possible result is complete removal of an electron from an atom • Hence all x-rays are ionizing radiation • If all the x-ray energy is given to an electron, it is called photoionization • If part of the energy is given to an electron and the remainder to a lower energy photon, it is called Compton scattering 31

Ultraviolet interactions • Near UV radiation (just shorter than visible wavelengths) is absorbed very strongly in the surface layer of the skin by electron transitions • At higher energies, ionization energies for many molecules are reached and the more dangerous photoionization processes occur • Sunburn is primarily an effect of UV radiation, and ionization produces 32 the risk of skin cancer

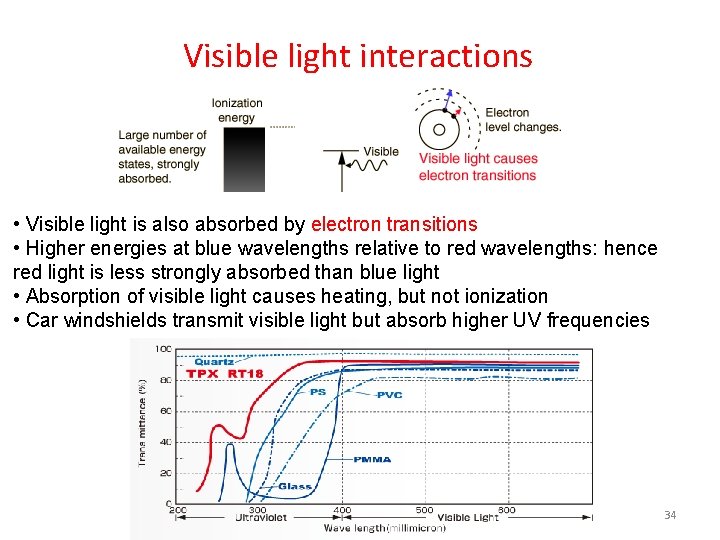
Visible light interactions • Visible light is also absorbed by electron transitions • Higher energies at blue wavelengths relative to red wavelengths: hence red light is less strongly absorbed than blue light • Absorption of visible light causes heating, but not ionization • Car windshields transmit visible light but absorb higher UV frequencies 34

Infrared (IR) interactions Vibrational transitions are associated with larger energies than ‘pure’ rotational transitions. Vibrations can be subdivided into two classes, depending on whether the bond length or angle is changing: • Stretching (symmetric and asymmetric) • Bending (scissoring, rocking, wagging and twisting) Stretching frequencies are higher than corresponding bending frequencies (it is easier to bend a bond than to stretch or compress it) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds 35

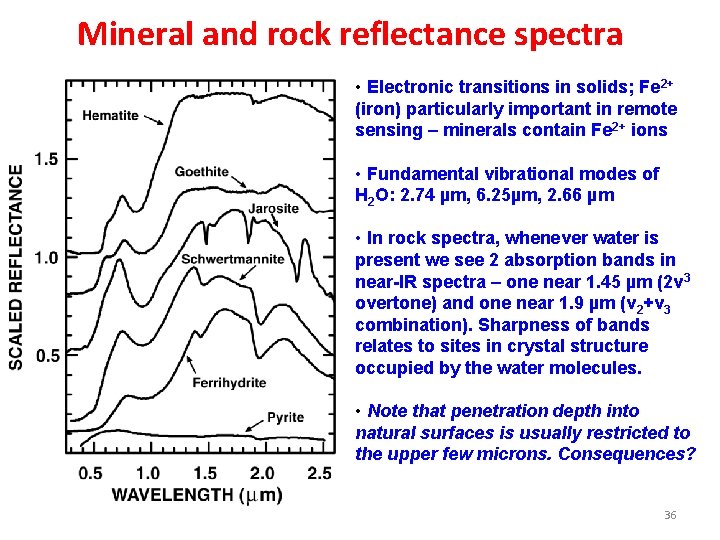
Mineral and rock reflectance spectra • Electronic transitions in solids; Fe 2+ (iron) particularly important in remote sensing – minerals contain Fe 2+ ions • Fundamental vibrational modes of H 2 O: 2. 74 µm, 6. 25µm, 2. 66 µm • In rock spectra, whenever water is present we see 2 absorption bands in near-IR spectra – one near 1. 45 µm (2ν 3 overtone) and one near 1. 9 µm (v 2+v 3 combination). Sharpness of bands relates to sites in crystal structure occupied by the water molecules. • Note that penetration depth into natural surfaces is usually restricted to the upper few microns. Consequences? 36

Geological mapping/prospecting Escondida Mine, Atacama Desert, Chile ASTER visible ASTER short-wave IR (SWIR)37

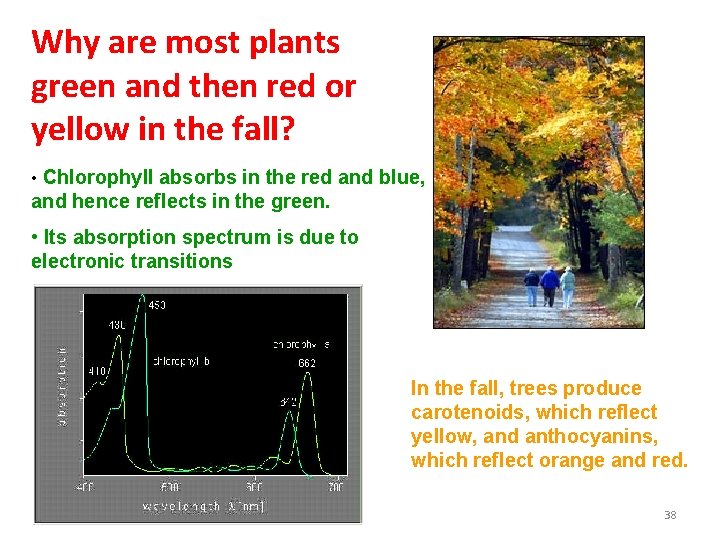
Why are most plants green and then red or yellow in the fall? • Chlorophyll absorbs in the red and blue, and hence reflects in the green. • Its absorption spectrum is due to electronic transitions In the fall, trees produce carotenoids, which reflect yellow, and anthocyanins, which reflect orange and red. 38

MOLECULAR SPECTRA The rotational, vibrational and electronic energy levels of a molecule are collectively called molecular energy levels. the transitions of energies can take place only between these levels. the result is a molecular spectrum. 39

TYPES OF MOLECULAR ENERGIES AND BORN OPPENHEIMER APPROXIMATION • • A molecule usually possesses four different types of energies. These are… Translational energy Rotational energy Vibrational energy Electronic energy • According to born oppenheimer approximation, the total energy of a molecule is the sum of translational , rotational, vibrational and electronic energies. E = E t + Er + Ev + Ee It is found that the translational energy is neglegibly small. So 40 E = Ev + Er + Ee

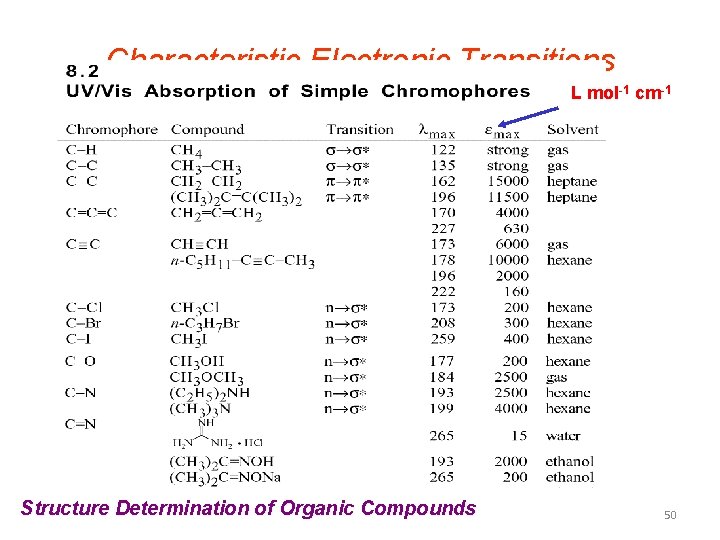
TYPES OF MOLECULAR SPECTRA • The energy absorbed for any transition is equal to the difference in the energies of the two levels involved. It is found that these energies for transition are in order… • Et << Er << Ev << Ee • Translational energy is considered as continous and we donot observe any translational spectrum. 41

• Pure rotational (microwave) spectra these spectra are observed in far infra red region or in the microwave region. • Vibrational rotational spectra such energies are available in the near infra red region. • Electronic band spectra for a given electronic transition, a set of bands observed. This set of bands is called a band group or a band system. Thus whereas atoms give line spectra, molecules give band spectra. These spectra are observed in the visible region and ultra violet region. 42

• Raman spectra this is also a type of vibrational rotational spectrum. It is based on scattering of radiation and not on the absorption of radiation by the sample. Raman spectra is observed in the visible region. • Nuclear magnetic resonance(nmr) spectra this type of spectra arises from the transition between the nuclear spin energy levels of the molecule when an external field is applied on it. The energies involved in these transitions are very high which lie in the radio frequency regions. • Electron spin resonance (esr) spectra this type of spectrum arise from the transitions between the electron spin energy levels of the molecule when an external magnetic field is applied on it. 43

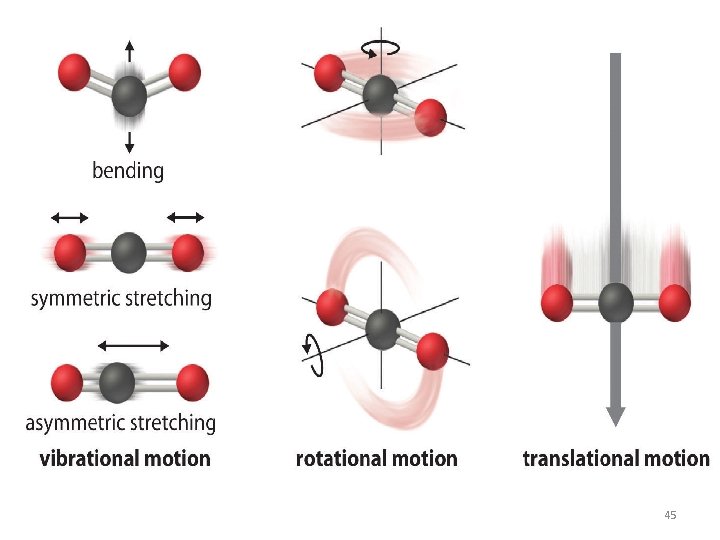
• Molecular Degrees of Freedom • The atoms, molecules, or ions that compose a chemical system can undergo several types of molecular motion, including translation, rotation, and vibration. • Translational motion. The entire molecule can move in some direction in three dimensions • Rotational motion. The entire molecule can rotate around any axis, (even though it may not actually change its position translationally) • Vibrational motion. The atoms within a molecule have certain freedom of movement relative to each other; this displacement can be periodic motion like the vibration of a tuning fork 44

45

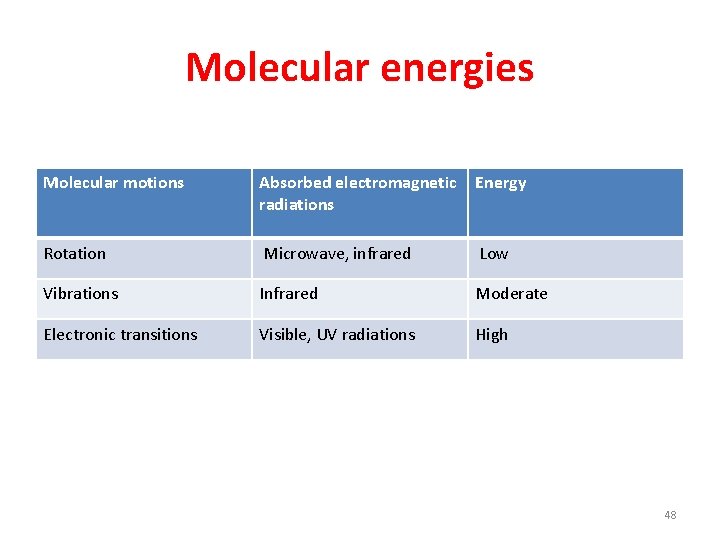
Molecular energies • An isolated molecule possesses: • translational energy by virtue of the motion of the molecule as a whole • (ii) rotational energy due to bodily rotation of the molecule about an axis passing through the center of gravity of molecule. • (iii) vibrational energy due to periodic displacement of its atoms from their equilibrium positions. • (iv) electronic energy since the electrons associated with each atom and bond are in constant motion. • the total energy of a molecule can be expressed • as the sum of the constituent energies, that is • Etotal = Etrans + Erot + Evib + Eelec 46

• The electrons and nuclei of atoms and molecules may exist in only certain specific energy levels, that is, they area quantized. They can absorb only photons having certain energies or wavelengths. • The energies of light absorbed by a molecule can be related to motions (energy modes) of the • molecule. A few examples are shown in Table I. 47

Molecular energies Molecular motions Absorbed electromagnetic radiations Energy Rotation Microwave, infrared Low Vibrations Infrared Moderate Electronic transitions Visible, UV radiations High 48

Radiations and Transitions Change of Spin 49

Characteristic Electronic Transitions L mol-1 cm-1 Structure Determination of Organic Compounds 50

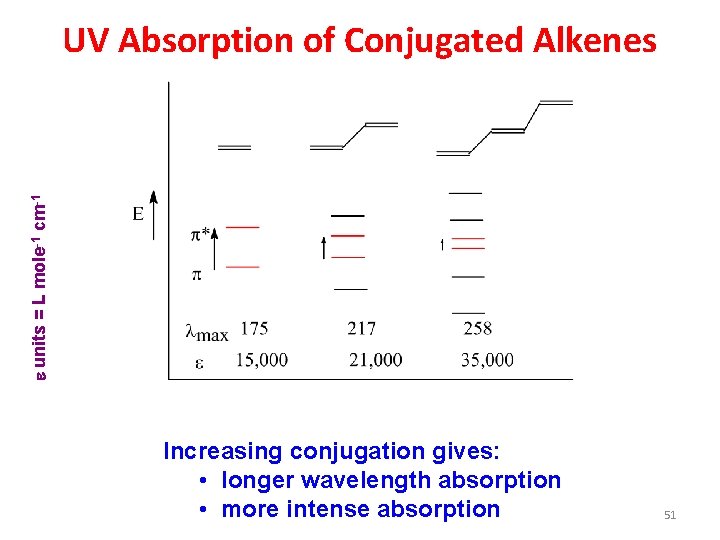
e units = L mole-1 cm-1 UV Absorption of Conjugated Alkenes Increasing conjugation gives: • longer wavelength absorption • more intense absorption 51

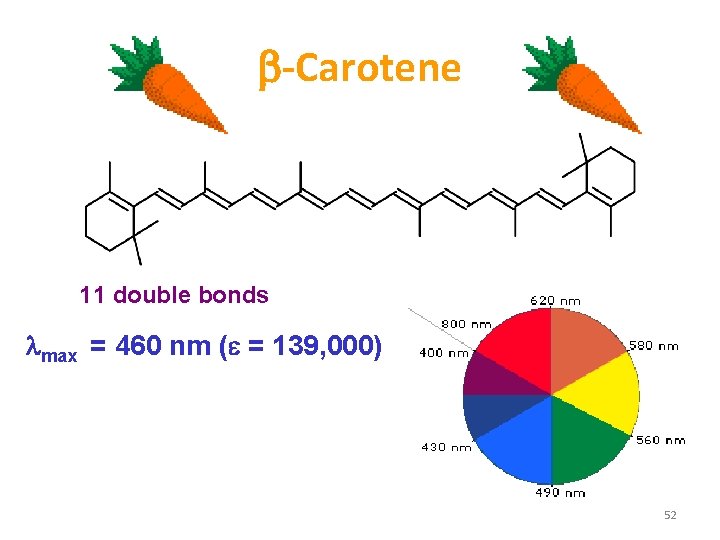
b Carotene 11 double bonds max = 460 nm (e = 139, 000) 52

Application of Spectroscopy in Petroleum Hydrocarbons / Oil Detection with UV/Vis Spectrometry Detection of the presence of hydrocarbons and oils in water is an important application in some industrial waste waters (petrochemical applications, but also food industry, e. g. in edible oil production) as well as in environmental monitoring (river, lake, seawater). Hydrocarbons and oil are groups of substances and should thus be considered sum parameters. However, they do not represent the total amount of organic materials present in the water, but a more specific fraction. 53

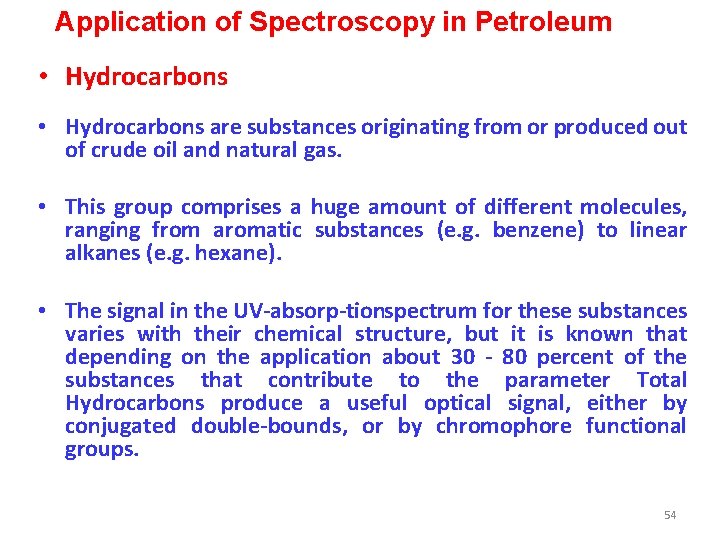
Application of Spectroscopy in Petroleum • Hydrocarbons are substances originating from or produced out of crude oil and natural gas. • This group comprises a huge amount of different molecules, ranging from aromatic substances (e. g. benzene) to linear alkanes (e. g. hexane). • The signal in the UV absorp tionspectrum for these substances varies with their chemical structure, but it is known that depending on the application about 30 80 percent of the substances that contribute to the parameter Total Hydrocarbons produce a useful optical signal, either by conjugated double bounds, or by chromophore functional groups. 54

Application of Spectroscopy in Petroleum The signal in the UV-absorption spectrum for hydrocarbons varies with their chemical structure. Depending on the application about 30 - 80 % of the substances produce a useful optical signal. 55

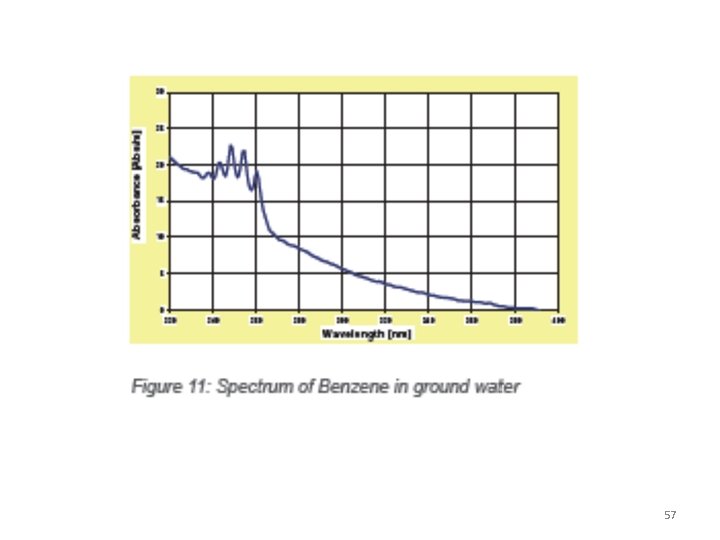
Application of Spectroscopy in Petroleum • Aromatic compounds • Common aromatic compounds present in crude oils in clude benzene , alkylbenzenes, alkylnaphthalenes, biphenyl, and aromatic steranes, all of them well detect able in the low ppb range in practical applications except highly turbid or strongly colored waters. • The polycyclic aromatic hydrocarbons, which are rated high priority pollutants because of their toxicity and their ubiquity. 56

57

Application of Spectroscopy in Petroleum • The possibility of measurement of hydrocar bons / oil in water using UV/Vis spectrometry strongly depends on the composition of the oil, and more specifically on the following points: • aromatic content > responsible for the main absorption signal • size of the molecules > determines miscibility 58

IMPORTANCE OF MOLECULAR SPECTROSCOPY • Molecular spectroscopy helps us to give electronic structure of the molecule and thus explain the various possible reactions that can take place. • Spectroscopic techniques are some of the most widely used analytical methods in the world today. • understanding of the molecular processes, molecular interactions, chemical system 59

Summary • Study of the detailed molecular structure through interaction of light with sample is known as spectroscopy • Light is EMR, consisting of various regions, depending upon different energies, each region is capable of interaction • Interaction causes molecular changes by absorption/emission of light • This produces a spectrum, which gives identification and analysis 60

61