SPECTROSCOPY SPECTROSCOPY Spectroscopy is the study of the









- Slides: 26

SPECTROSCOPY -

SPECTROSCOPY: Spectroscopy is the study of the interaction between electromagnetic radiation and matter. The matter can be atoms, molecules or ions SPECTRUM Spectrum is a plot of the amount of light absorbed by a sample versus the wavelength of the light. A plot of the response as a function of wavelength or more commonly frequency is referred to as a spectrum. • The amount of light absorbed is called the absorbance. SPECTROMETRY: It is the measurement of these responses and an instrument which performs such measurements is a spectrometer or spectrograph, although these terms are more limited in use to the original field of optics from which the concept sprang. Spectroscopy is often used in physical and analytical chemistry for the identification of substances through the spectrum emitted from or absorbed by them. Spectroscopy is also heavily used in astronomy and remote sensing. Most large telescopes have spectrometers, which are used either to measure the chemical composition and physical properties of astronomical objects or to measure their velocities from the Doppler Shift of their spectral lines.



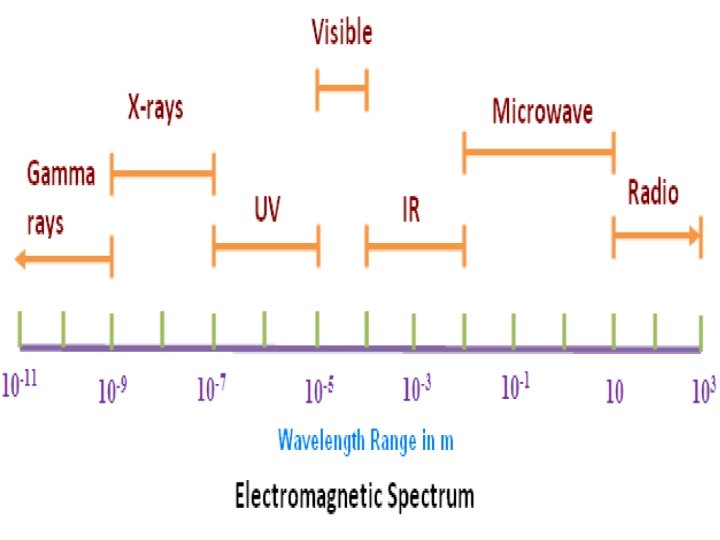
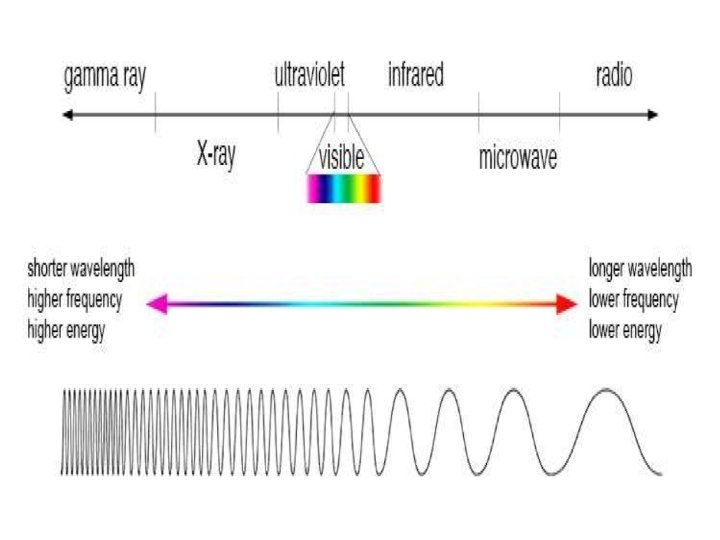
Interaction with matter. . . • The effect of electromagnetic radiation on interaction with matter depends on energy associated with the radiation • Very energetic radiations (UV and x-ray) may cause an electron to be ejected from the molecules • Radiation in the infrared region of the spectrum have much less energy they can cause vibrations in molecules • Microwave radiation is even less energetic than infrared radiation it can neither induce electronic transition in molecules nor can it cause vibrations it can only cause molecules to rotate

Classification of Methods � The type of spectroscopy depends on the physical quantity measured. Normally, the quantity that is measured is an intensity, either of energy absorbed or produced. � Most spectroscopic methods are differentiated as either atomic or molecular based on whether or not they apply to atoms or molecules. Along with that distinction, they can be classified on the nature of their interaction: Ø ABSORPTION SPECTROSCOPY It uses the range of the electromagnetic spectra in which a substance absorbs. This includes atomic absorption spectroscopy and various molecular techniques, such as infra-red spectroscopy in that region and Nuclear Magnetic resonance spectroscopy in the radio region.

Classification of Methods EMISSION SPECTROSCOPY It uses the range of electromagnetic spectra in which a substance radiates (emits). The substance first must absorb energy. This energy can be from a variety of sources, which determines the name of the subsequent emission, like luminescence. Molecular luminescence techniques include spectroflourimetry. Ø SCATTERING SPECTROSCOPY It measures the amount of light that a substance scatters at certain wavelengths, incident angles, and polarization angles. The scattering process is much faster than the absorption/emission process. One of the most useful applications of light scattering spectroscopy is Raman Spectroscopy. Ø

Principles of absorption spectroscopy � Lamberts law: It states that when monochromatic light passes through a transparent medium, the intensity of transmitted light decreases exponentially as the thickness of absorbing material increases � Beer’s law: It stats that the intensity of transmitted monochromatic light decreases exponentially as the concentration of the absorbing substance increases Ø Beer-lamberts law: The Beer-Lambert law states that the amount of light absorbed by a substance dissolved in a fully transmitting solvent is directly proportional to the concentration of the substance and the path length.

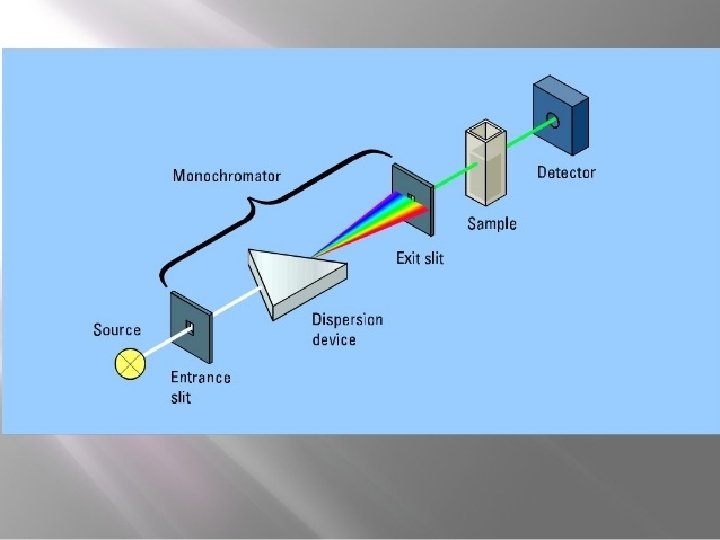
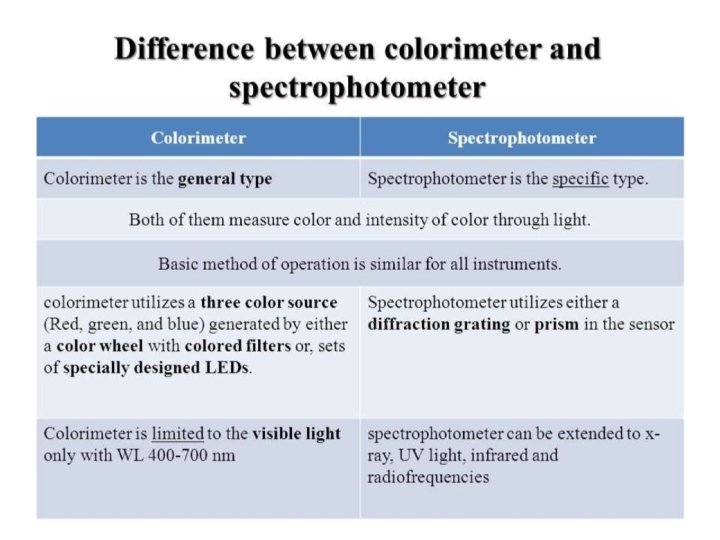
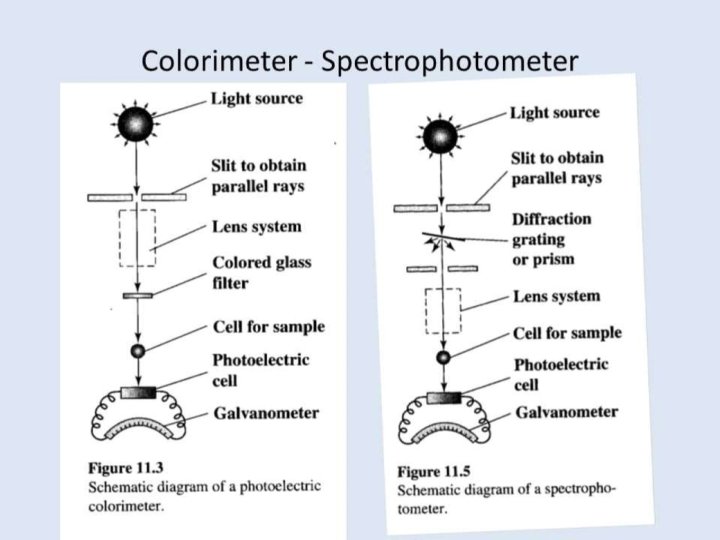
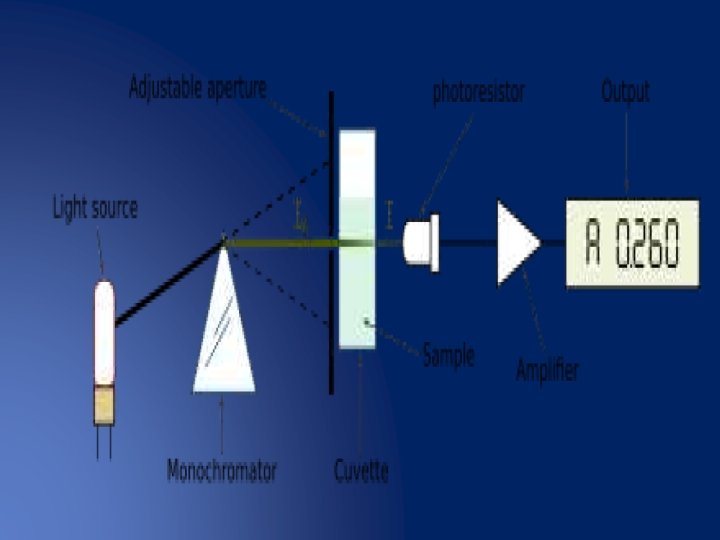
Spectrophotometer. . . Ø A spectrophotometer is an instrument that measures the amount of light absorbed by a sample. Ø Used to measure the concentration of solutes in solution by measuring the amount of the light that is absorbed by the solution in a cuvette placed in the spectrophotometer. o PARTS : - • • Light source A monochromator Sample holder(cuvvette) Light detector

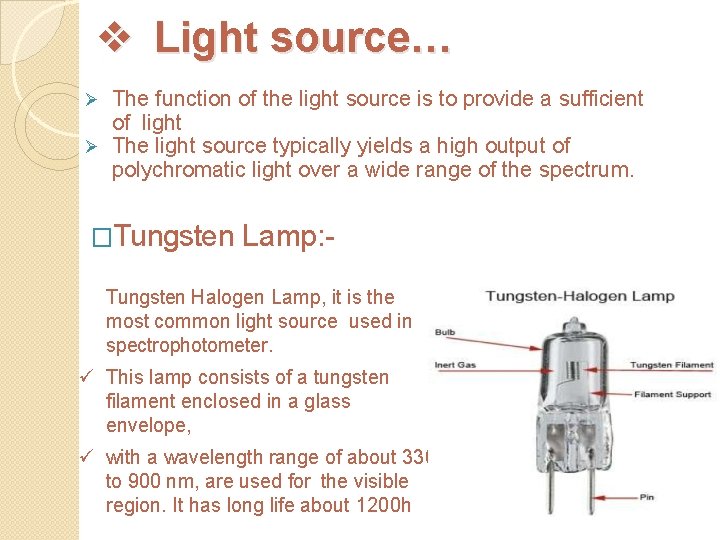
v Light source… The function of the light source is to provide a sufficient of light Ø The light source typically yields a high output of polychromatic light over a wide range of the spectrum. Ø �Tungsten Lamp: - ü Tungsten Halogen Lamp, it is the most common light source used in spectrophotometer. ü This lamp consists of a tungsten filament enclosed in a glass envelope, ü with a wavelength range of about 330 to 900 nm, are used for the visible region. It has long life about 1200 h

Hydrogen / Deuterium Lamps For the ultraviolet region, hydrogen or deuterium lamps are frequently used. � Their range is approximately 200 to 450 nm. � Deuterium lamps are generally more stableand has long life about 500 h. � This lamp generates continuous or discontinuous spectral. � Xenon flash lamps • Their range between (190 nm 1000 nm) • Emit both UV and visible wavelengths • • • Long life Do not heat up the instrument Reduce warm up time

v. Monochromator • • • Monochromator Accepts polychromatic input light from a lamp and outputs monochromatic light. Monochromator consists of three parts: I) Entrance slit II) Exit slit III) Dispersion device v. Dispersion devices • Dispersion devices causes a different wavelength of light to be dispersion at different angles monochromators used for function. Ø Types of dispersion devices : • Prism: - is used to isolate different wavelength. • • If a parallel beam of radiation falls on a prism , the radiation of two different wavelength will be bent through different angles. Prism may be made of glass or quartz. The glass prisms are suitable for radiation essentially in the visible range whereas the quartz prism can cover the ultraviolet spectrum also.

v. Filters separate different parts of the electromagnetic spectrum by absorbing or reflecting certain wavelengths and transmitting other wavelengths. • Absorption filters: �are glass substrates containing absorbing species that absorb certain wavelength. • Interference filters: �are made of multiple dielectric thin films on a substrate. • They use interference to selectively transmit or reflect a certain range of wavelengths.

v. Absorption cells(Cuvettes) A cuvette is a kind of cell (usually a small square tube) sealed at one end • Made of Plastic, glass or optical grade quartz • Designed to hold samples for spectroscopic experiments. • Cuvette should be as clear as possible, without impurities that might affect a •




Types of spectrophotometer 1. Single beam spectrophotometer 2. Double beam spectrophotometer 3. Split beam spectrophotometer Single beam spectrophotometer : • • • The single beam spectrophotometer was the first invented, all the light passes through the sample. In this case, to measure the intensity of the incident light, the sample must be removed so all the light can pass through. • This type is cheaper because there are less parts and the • • system is less complicated. low cost, high Sensitivity , because the optical system is simple. • The disadvantage is that an appreciable amount of Time elapses between taking the reference and Making the sample measurement


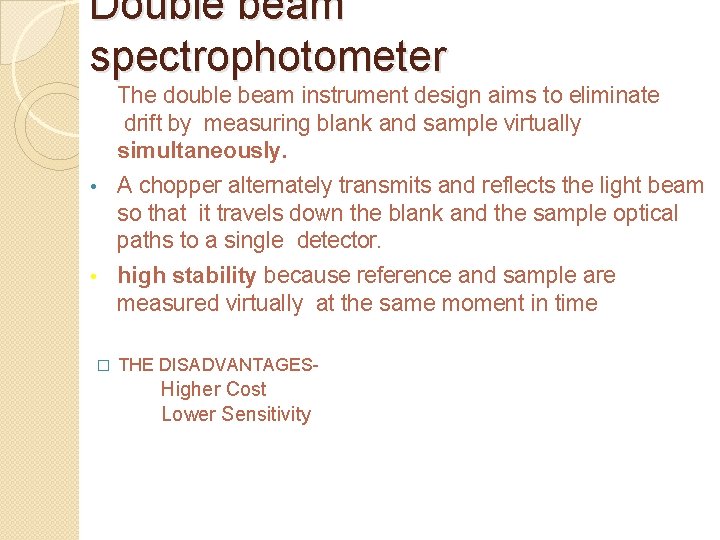
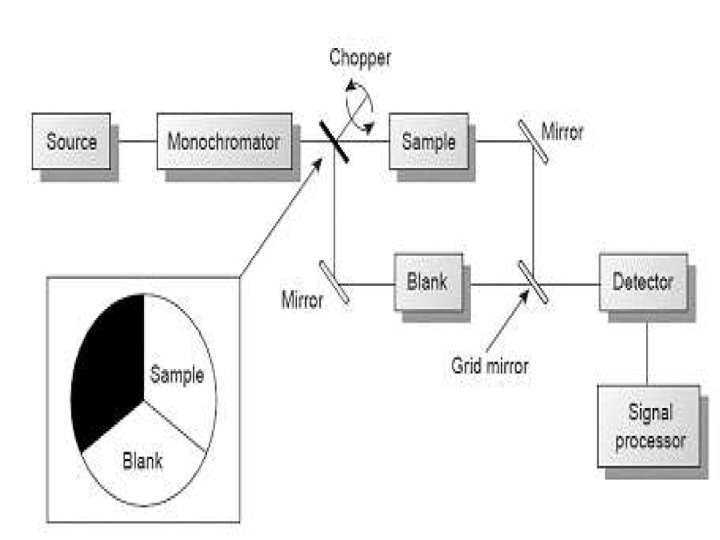
Double beam spectrophotometer • The double beam instrument design aims to eliminate drift by measuring blank and sample virtually simultaneously. • A chopper alternately transmits and reflects the light beam so that it travels down the blank and the sample optical paths to a single detector. • high stability because reference and sample are measured virtually at the same moment in time � THE DISADVANTAGES- Higher Cost Lower Sensitivity


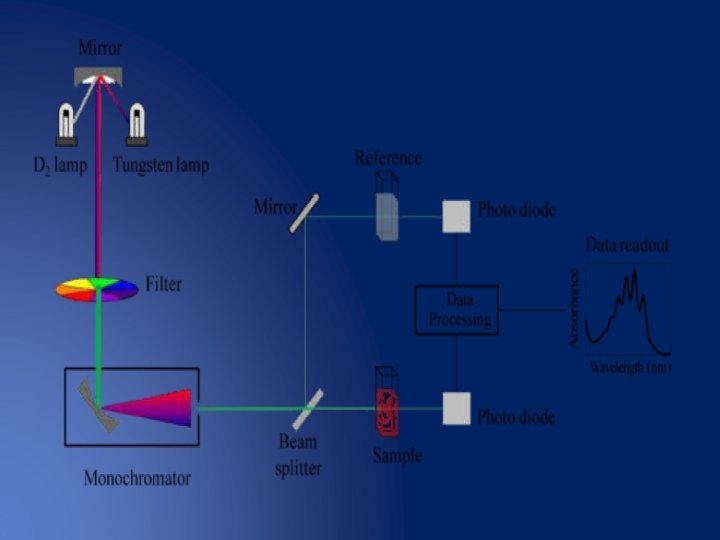
Split beam Spectrophotometer The split beam spectrophotometer is similar to the double beam spectrophotometer but it uses a beam splitter instead of a chopper. • Thus blank and sample measurements can be made at the same moment in time. • Spectra are measured in the same way as with a double beam spectrophotometer. • The advantage of this design is good stability. •


Other Common Types. . 1 - Fluorescence spectroscopy: Fluorescence spectroscopy uses higher energy photons to excite a sample, which will then emit lower energy photons. This technique has become popular for its biochemical and medical applications. Ø 2 - X-ray spectroscopy: X-rays of sufficient frequencies interact with material and excite the atoms contained. Due to this excitation Auger Effect is produced and some excitation radiations are absorbed or evolved if vice versa occurs. Itis used in chemistry and material sciences to determine elemental composition and chemical bonding. Ø 3 -Flame Spectroscopy: Liquid solution samples are aspirated into a burner or nebulizer/burner combination, desolvated, atomized, and sometimes excited to a higher energy electronic state. The use of a flame during analysis requires fuel and oxidant, typically in the form of gases. Ø

4 - Spark or arc (emission) spectroscopy: Since the conditions producing the arc emission typically are not controlled quantitatively, the analysis for the elements is qualitative. Nowadays, the spark sources with controlled discharges under an argon atmosphere allow that this method can be considered eminently quantitative, and its use is widely expanded worldwide through production control laboratories of foundries and steel mills. Ø 5 - UV/VIS spectroscopy: Ø It basically involves the spectroscopy of photons and spectrophotometery. It uses light in the visible and adjacent near ultraviolet (UV) and near infrared (NIR) ranges. It used in the quantitative determination of solutions of transition metal ions and highly conjugated Ø 6 - Infra-red Spectroscopy: (IR spectroscopy) is the subset of spectroscopy that deals with the infrared region of the electromagnetic spectrum. Especially in organic chemistry the analysis of IR absorption spectra shows what type of bonds are present in the sample. Ø 7 - Raman Spectroscopy: It relies on inelastic scattering, or Raman scattering of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range.

THANK YOU