Molecular Shape VSEPR Model Molecular Shape PhysicalChemical PROPERTIES





- Slides: 20

Molecular Shape VSEPR Model

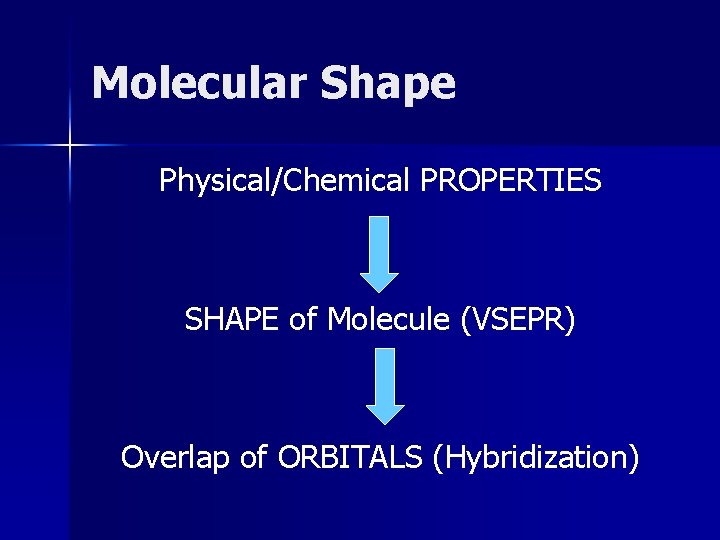
Molecular Shape Physical/Chemical PROPERTIES SHAPE of Molecule (VSEPR) Overlap of ORBITALS (Hybridization)

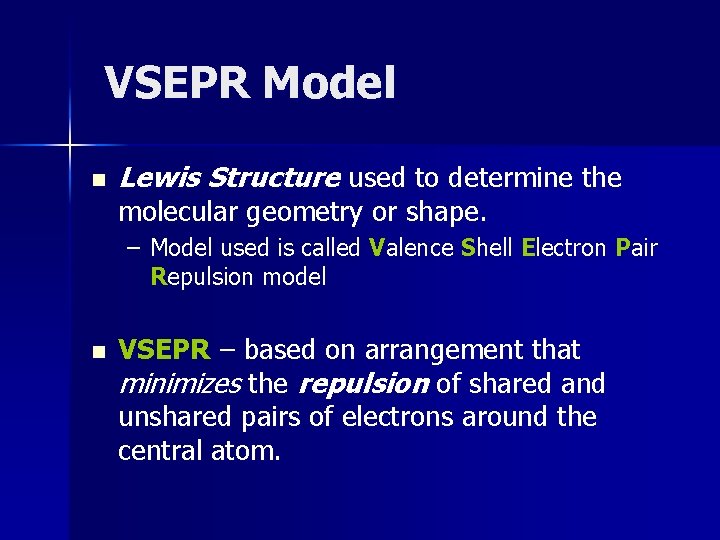
VSEPR Model n Lewis Structure used to determine the molecular geometry or shape. – Model used is called Valence Shell Electron Pair Repulsion model n VSEPR – based on arrangement that minimizes the repulsion of shared and unshared pairs of electrons around the central atom.

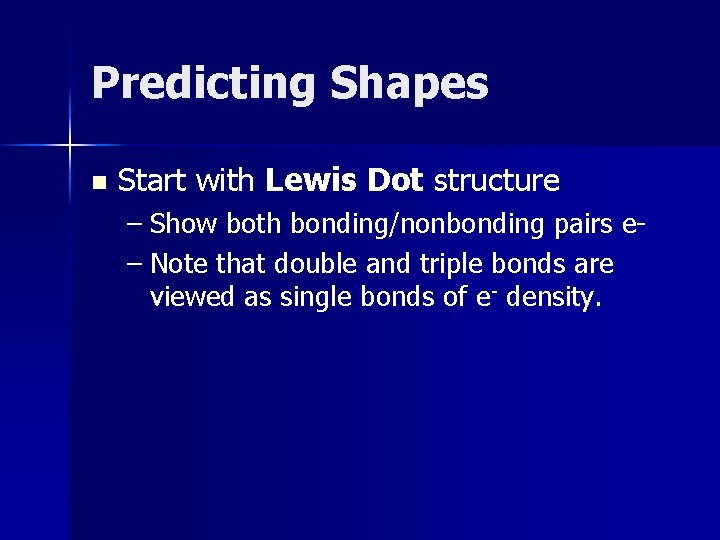
Predicting Shapes n Start with Lewis Dot structure – Show both bonding/nonbonding pairs e– Note that double and triple bonds are viewed as single bonds of e- density.

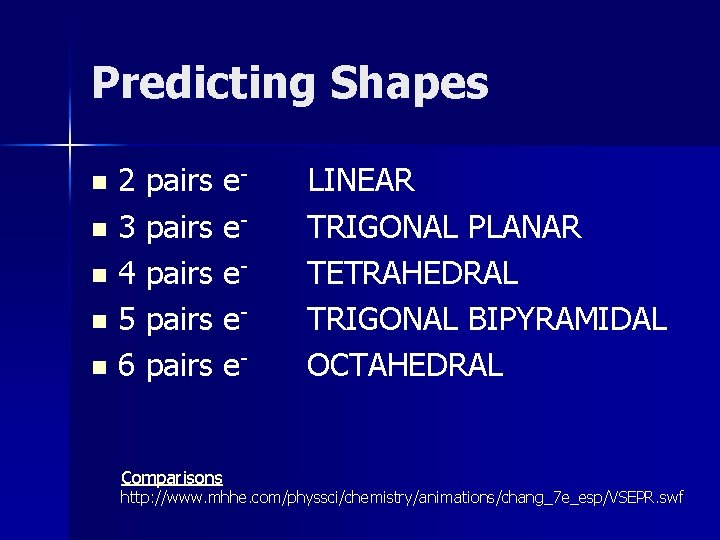
Predicting Shapes 2 pairs en 3 pairs en 4 pairs en 5 pairs en 6 pairs en Comparisons LINEAR TRIGONAL PLANAR TETRAHEDRAL TRIGONAL BIPYRAMIDAL OCTAHEDRAL http: //www. mhhe. com/physsci/chemistry/animations/chang_7 e_esp/VSEPR. swf

Molecular Shapes (routine) n Draw Lewis Structure – Focus on central atom n Count up the regions of electron density on central atom – i. e. , bonding and nonbonding pairs n Count up the number of atoms bonded to the central atom

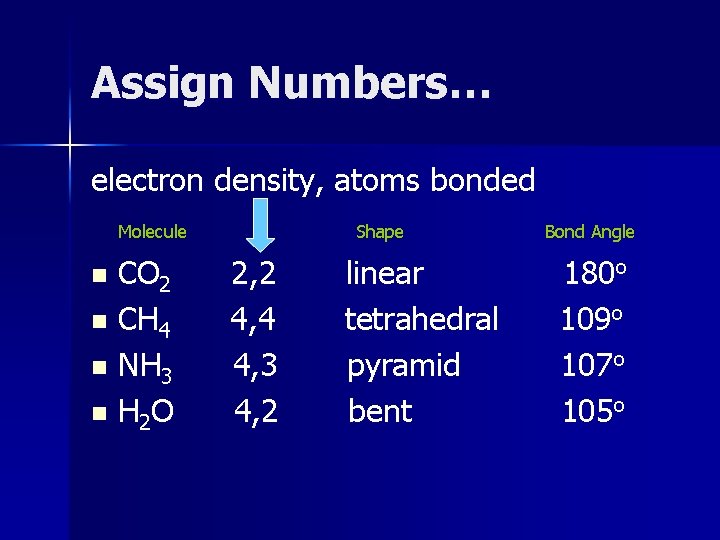
Assign Numbers… electron density, atoms bonded Molecule CO 2 n CH 4 n NH 3 n H 2 O n Shape 2, 2 4, 4 4, 3 4, 2 linear tetrahedral pyramid bent Bond Angle 180 o 109 o 107 o 105 o

Molecular Geometry n Examples http: //www. uwosh. edu/faculty_staff/xie/tutorial/vsepr. htm#live

Hybridization n Mixing of two or more atomic orbitals of similar energies on the same atom to give new orbitals or equal energies. – Special types of atomic orbitals that have energy intermediate between s and p orbitals. Animation http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/hybrv 18. swf More… http: //www. mhhe. com/physsci/chemistry/animations/chang_7 e_esp/bom 5 s 2_6. swf

sp 3 Hybridizaiton

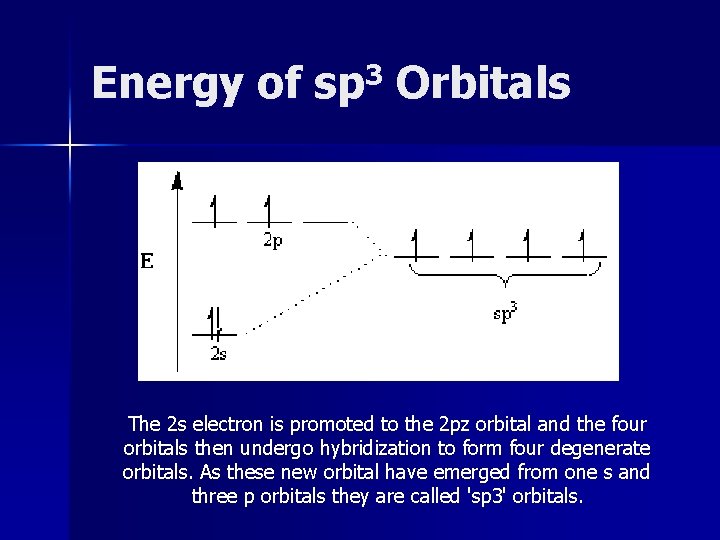
Energy of sp 3 Orbitals The 2 s electron is promoted to the 2 pz orbital and the four orbitals then undergo hybridization to form four degenerate orbitals. As these new orbital have emerged from one s and three p orbitals they are called 'sp 3' orbitals.

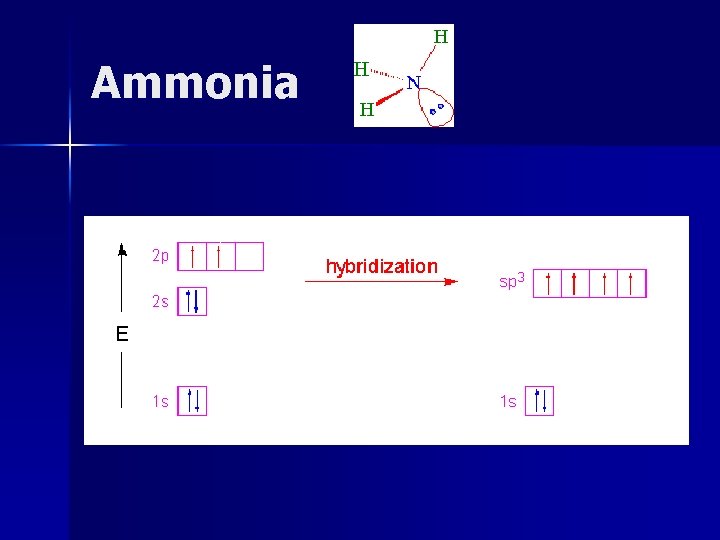
Ammonia bonding electrons in a probability area for the hybrid orbital. . .

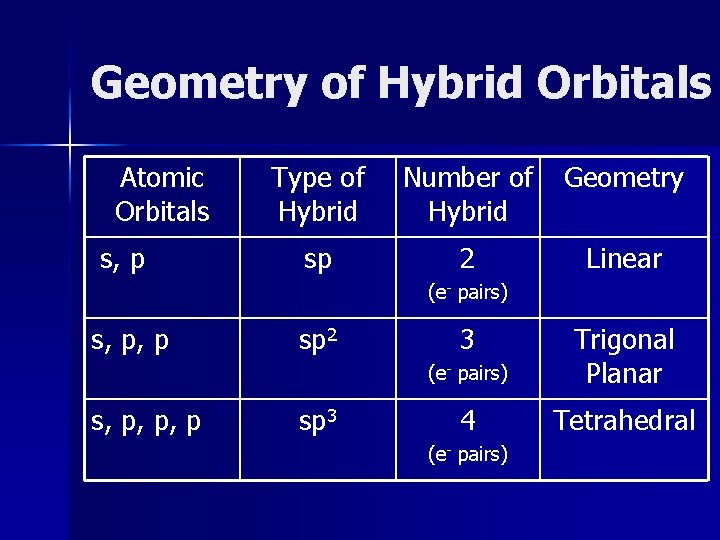
Geometry of Hybrid Orbitals Atomic Orbitals s, p Type of Hybrid Number of Hybrid Geometry sp 2 Linear (e- pairs) s, p, p, p sp 2 sp 3 3 (e- pairs) Trigonal Planar 4 Tetrahedral (e- pairs)

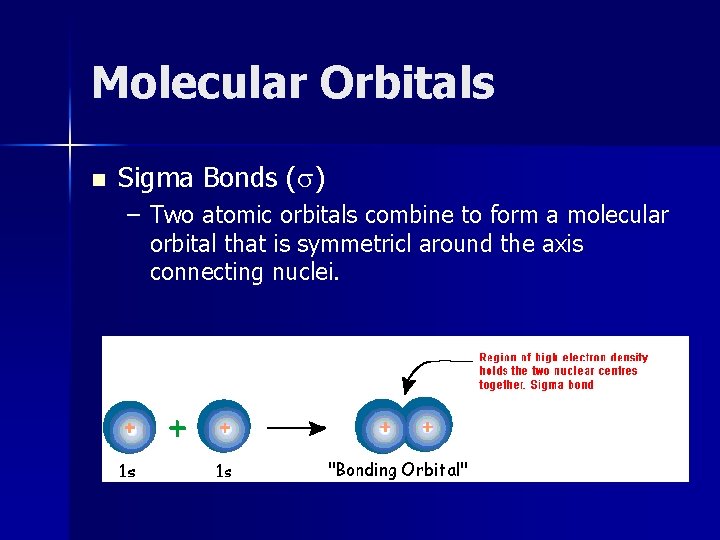
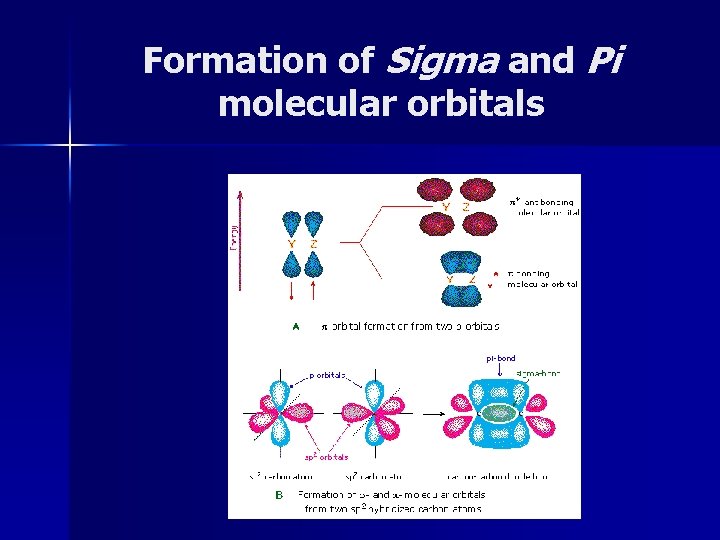
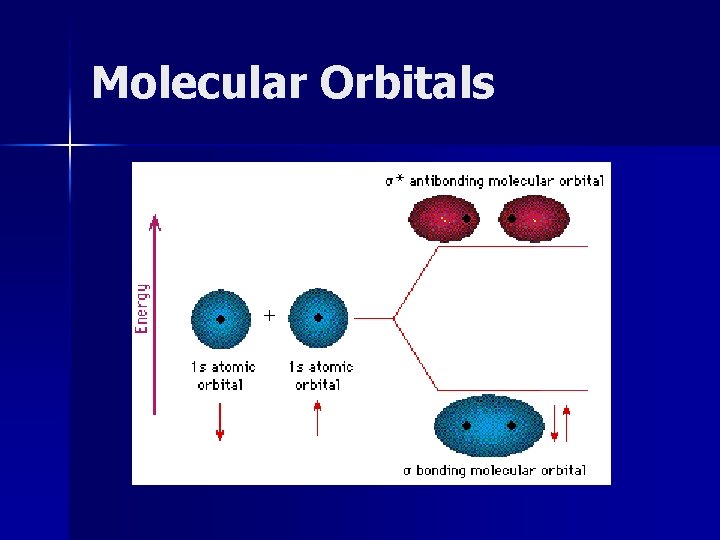
Molecular Orbitals n Sigma Bonds (s) – Two atomic orbitals combine to form a molecular orbital that is symmetricl around the axis connecting nuclei.

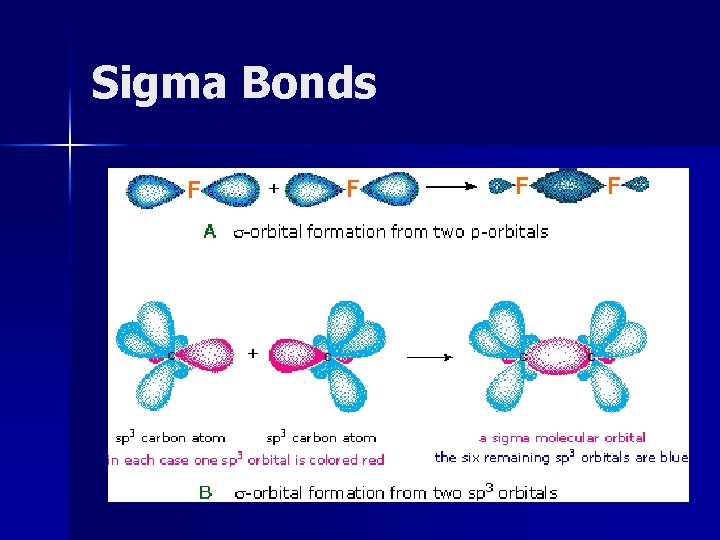
Sigma Bonds

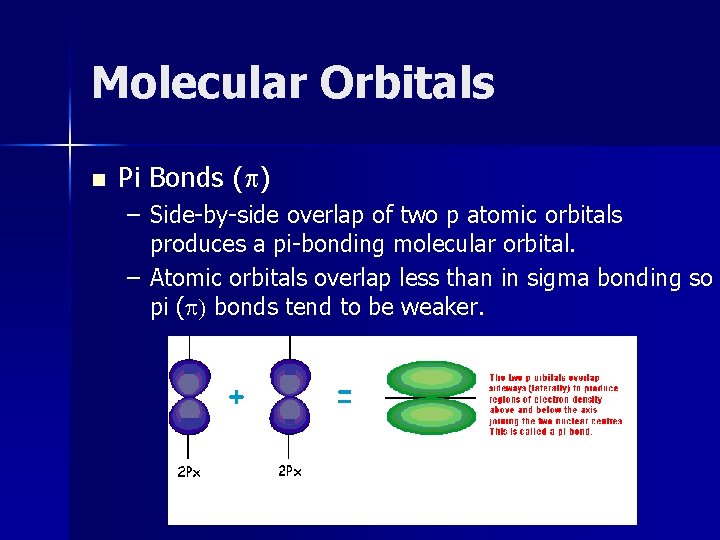
Molecular Orbitals n Pi Bonds (p) – Side-by-side overlap of two p atomic orbitals produces a pi-bonding molecular orbital. – Atomic orbitals overlap less than in sigma bonding so pi (p) bonds tend to be weaker.

Hybridization and Multiple Bonds n Maximum of two electrons per orbital – So multiple bonds must include sigma (s) and pi (p) bonds. Double Bonds One s and one p n Triple Bonds One s and two p n

Formation of Sigma and Pi molecular orbitals

Molecular Orbitals

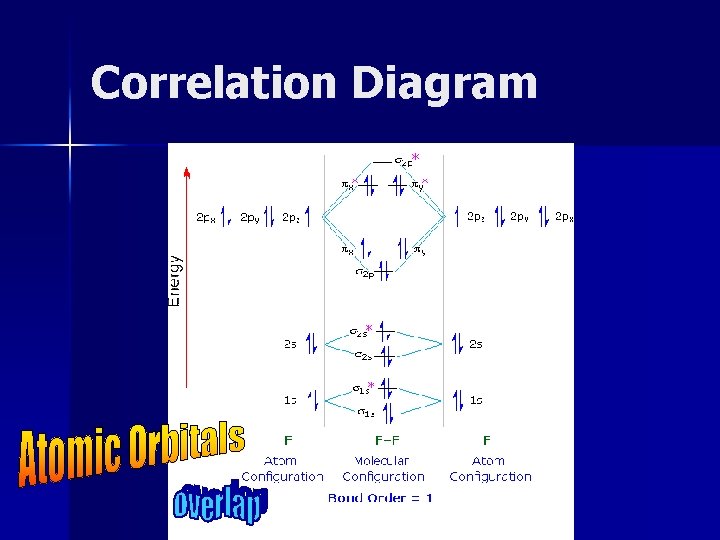
Correlation Diagram