Molecular compounds are made of just nonmetals smallest


















- Slides: 23


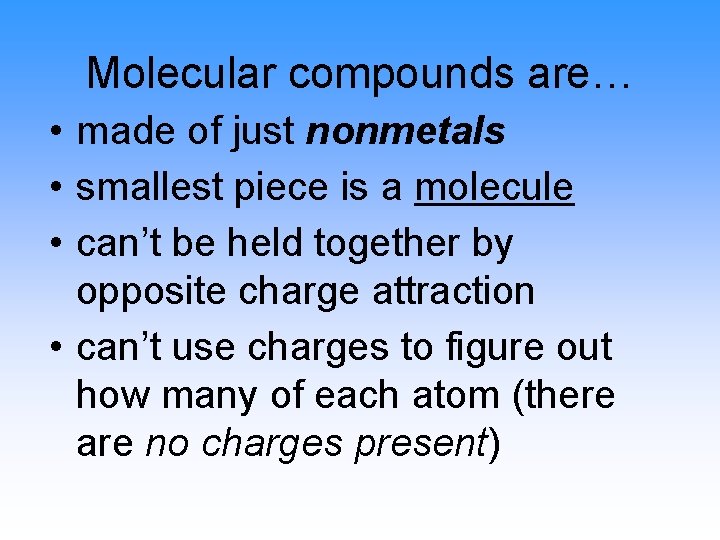
Molecular compounds are… • made of just nonmetals • smallest piece is a molecule • can’t be held together by opposite charge attraction • can’t use charges to figure out how many of each atom (there are no charges present)

Molecular compounds are easier! • Ionic compounds use charges to determine how many of each. – You have to figure out charges. – May need to criss-cross numbers. • Molecular compounds: the name tells you the number of atoms. –Uses prefixes to tell you the exact number of each element present!

Prefixes • • 1 = mono- • 9 = nona 2 = di • 10 = deca • To write the name, write two 3 = triwords: 4 = tetra 5 = penta- • Prefix & name Prefix name - ide 6 = hexa 7 = hepta 8 = octa-

Prefixes • One exception is we don’t write mono if there is only one of the first element. Prefix name -ide

Prefixes • 9 = nona • 10 = deca • To write the name, write two words: Prefix name -ide • One exception is we don’t write mono if there is only one of the first element. • Normally, we do not have double vowels when writing names (oa oo)

Practice by naming these: • • • N 2 O NO 2 Cl 2 O 7 CBr 4 CO 2 Ba. Cl 2 = dinitrogen monoxide(also called nitrous oxide or laughing gas) = nitrogen dioxide = dichlorine heptoxide = carbon tetrabromide = carbon dioxide (This one will not use prefixes, since it is an ionic compound!)

Write formulas for these: • • diphosphorus pentoxide tetraiodine nonoxide sulfur hexafluoride nitrogen trioxide carbon tetrahydride phosphorus trifluoride aluminum chloride (Ionic compound)



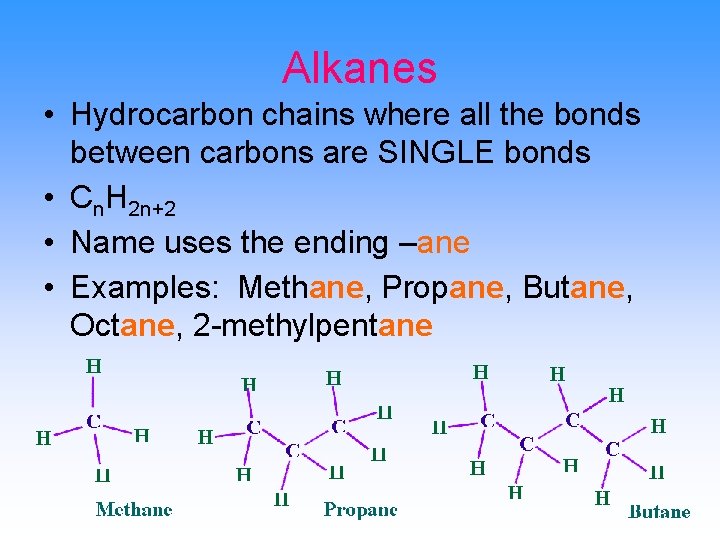
Alkanes • Hydrocarbon chains where all the bonds between carbons are SINGLE bonds • Cn. H 2 n+2 • Name uses the ending –ane • Examples: Methane, Propane, Butane, Octane, 2 -methylpentane

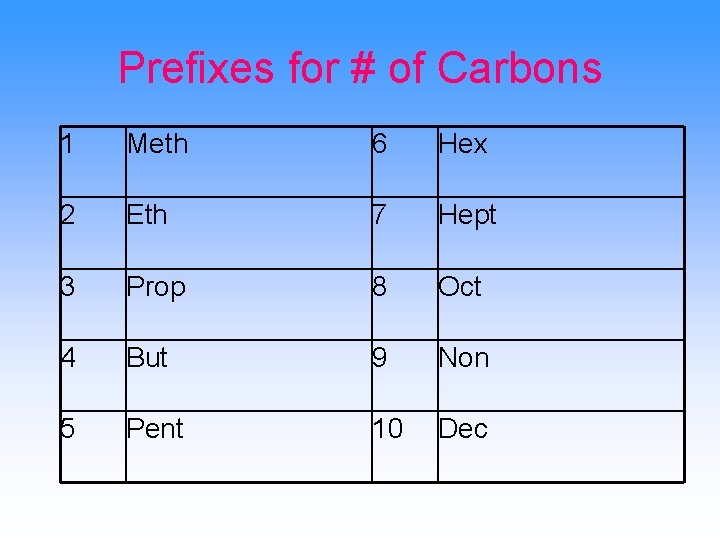
Prefixes for # of Carbons 1 Meth 6 Hex 2 Eth 7 Hept 3 Prop 8 Oct 4 But 9 Non 5 Pent 10 Dec

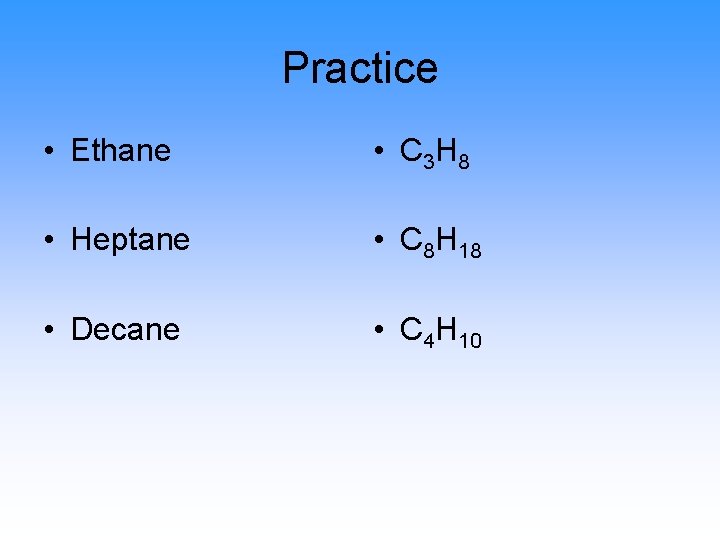
Practice • Ethane • C 3 H 8 • Heptane • C 8 H 18 • Decane • C 4 H 10

Acids are… • Compounds that give off hydrogen ions (H 1+) when dissolved in water (the Arrhenius definition) • Will start the formula with H. • There will always be some Hydrogen next to an anion. • The anion determines the name.

Rules for Naming acids: Name it as a normal compound first 1) If the anion attached to hydrogen ends in -ide, put the prefix hydro- and change -ide to -ic acid • HCl - hydrogen ion and chloride ion = hydrochloric acid • H 2 S hydrogen ion and sulfide ion = hydrosulfuric acid

Naming Acids If the anion has oxygen in it, then it ends in ate or -ite 2) change the suffix -ate to -ic acid (use no prefix) • Example: HNO 3 Hydrogen and nitrate ions = Nitric acid 3) change the suffix -ite to -ous acid (use no prefix) • Example: HNO 2 Hydrogen and nitrite ions = Nitrous acid •

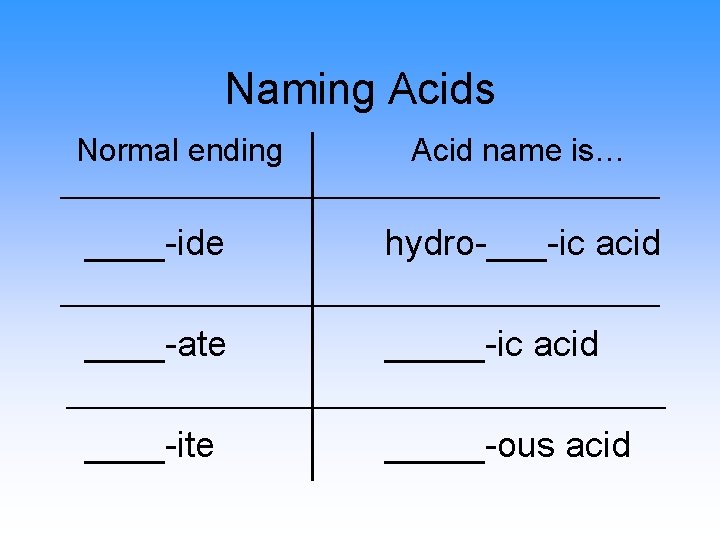
Naming Acids Normal ending Acid name is… ____-ide hydro-___-ic acid ____-ate _____-ic acid ____-ite _____-ous acid

Practice by naming these: • HF • H 3 P • H 2 SO 4 • H 2 SO 3 • HCN • H 2 Cr. O 4

Practice by naming these: • HF – hydrofluoric acid • H 3 P – hydrophosphoric acid • H 2 SO 4 - sulfuric acid • H 2 SO 3 - sulfurous acid • HCN – hydrocyanic acid • H 2 Cr. O 4 – chromic acid

Writing Acid Formulas – in reverse! • • Hydrogen will be listed first The name will tell you the anion Be sure the charges cancel out. Starts with prefix hydro? - there is no oxygen, -ide ending for anion • no prefix hydro? 1) -ate anion comes from –ic ending 2) -ite anion comes from –ous ending

Write formulas for these: • hydroiodic acid • acetic acid • carbonic acid • phosphorous acid • hydrobromic acid

Write formulas for these: • hydroiodic acid - HI • acetic acid - HC 2 H 3 O 2 • carbonic acid - H 2 CO 3 • phosphorous acid - H 3 PO 3 • hydrobromic acid - HBr

 Mikael ferm
Mikael ferm Examples of binary molecular compounds
Examples of binary molecular compounds Naming binary molecular compounds
Naming binary molecular compounds Binary molecular compounds are made of two
Binary molecular compounds are made of two Non metals examples
Non metals examples Metals react with nonmetals to form ionic compounds by
Metals react with nonmetals to form ionic compounds by How to name 2 nonmetals
How to name 2 nonmetals Compounds formed between metal and nonmetals
Compounds formed between metal and nonmetals Ionic and covalent bonds venn diagram
Ionic and covalent bonds venn diagram Naming molecular compounds
Naming molecular compounds Naming compounds and writing formulas
Naming compounds and writing formulas Naming molecular compounds
Naming molecular compounds Binary molecular compounds
Binary molecular compounds Binary molecular compounds def
Binary molecular compounds def Molecular compounds def
Molecular compounds def Compound vs molecule
Compound vs molecule Low molecular compounds
Low molecular compounds Chemical formula for pentasulfur dinitride
Chemical formula for pentasulfur dinitride Naming molecular compounds
Naming molecular compounds Nbr5 compound name
Nbr5 compound name Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Why does juliet note that romeo’s lips are warm?
Why does juliet note that romeo’s lips are warm?